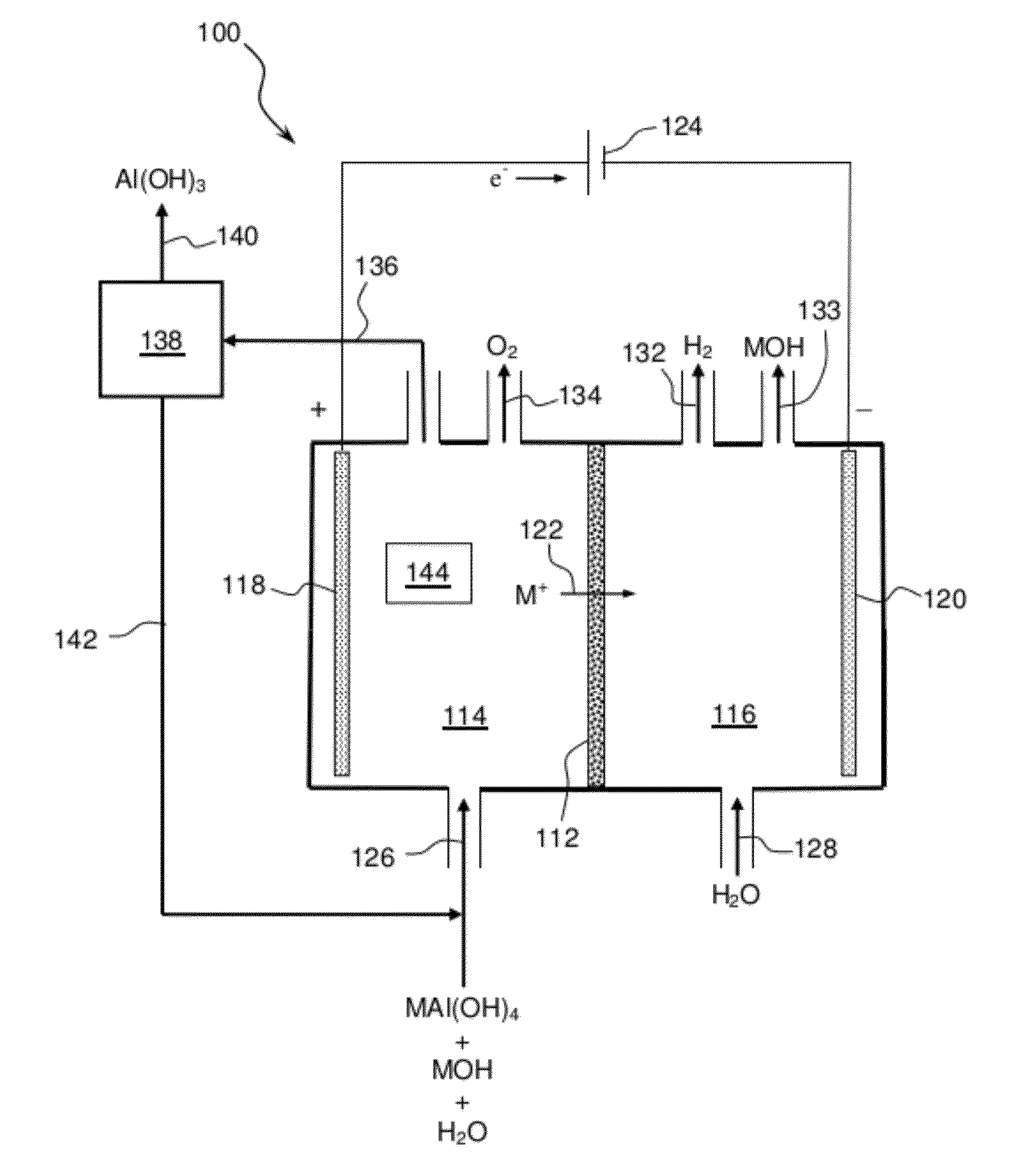
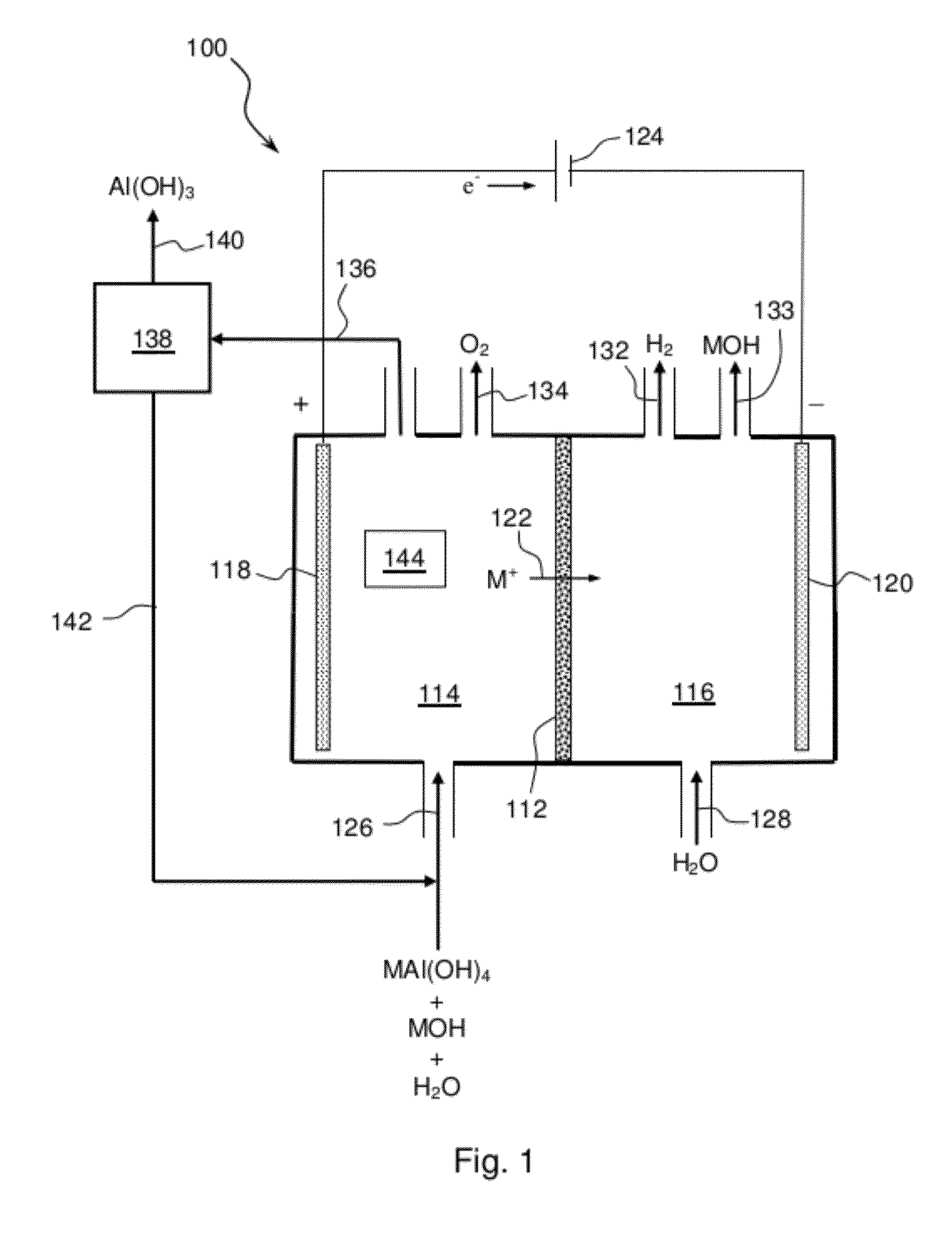
Electrolytic process to produce aluminum hydroxide
a technology of electrolysis and aluminum hydroxide, which is applied in the direction of electrolysis components, energy inputs, aluminium oxides/hydroxides, etc., can solve the problems of difficult recovery of valuable aluminum and alkali metal compounds from industrial waste streams containing alkali aluminate, and achieve the effect of increasing the efficiency of the apparatus and method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
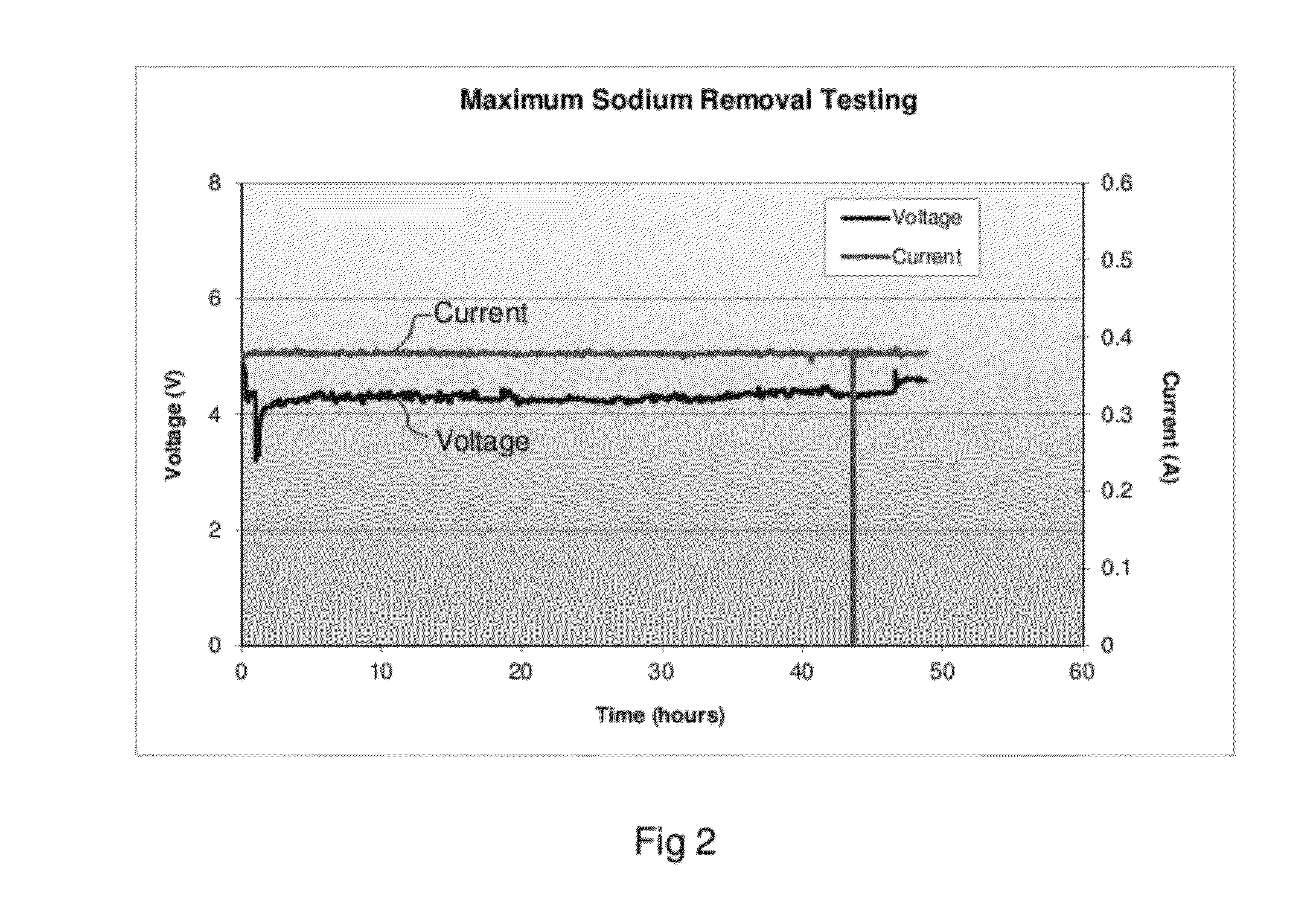
[0052]A solution containing 5.64 molarity of NaOH in solution sodium aluminate waste stream was heated to 40° C. as the anolyte. The anode was Kovar (Fe—Ni—Co) and the cathode was Kovar. The cell was operated in a batch mode of operation at a current density of 75 mA per sq.cm. of membrane area. The initial catholyte was 1M NaOH. FIG. 2 is a plot which presents the sodium transfer current-versus voltage to drive sodium across the two compartment cell to thereby separate sodium from sodium aluminate.
[0053]The voltage remained between 4 to 5 volts during the entire duration for majority of the test. It should be noted that the cell was operated for a known duration in batch mode to establish cell performance only. A total of 82.7% of sodium was separated from the sodium aluminate sample in this test as determined by ICP analysis. FIG. 3 shows samples collected at different level of sodium separation with membrane cell and formation of aluminum hydroxide. The level of sodium separation...
example 2
[0058]A NaSICON membrane was assembled in a two-compartment cell configuration and operated an in electrochemical cell with anolyte and catholyte solutions. Operated at constant current density of 75 mA / cm2, several batch tests were conducted to demonstrate the approach to produce sodium hydroxide and aluminum hydroxide from the waste sodium aluminate based sample. The electrolytic cell was operated for about 20 hours at 40° C. The initial and final anolyte and catholyte solutions were submitted for sodium mass balance analysis to determine the sodium concentration. The average power consumption to make NaOH was determined from the sodium mass balance analysis results.
[0059]FIG. 6 is a plot which presents the sodium transfer at constant current, the voltage is the potential required to drive sodium across the two compartment cell operated in batch mode as a function of time to thereby separate sodium from sodium aluminate in multiple batch testing. The voltage remained between 4 to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| conductive | aaaaa | aaaaa |
| electric current | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com