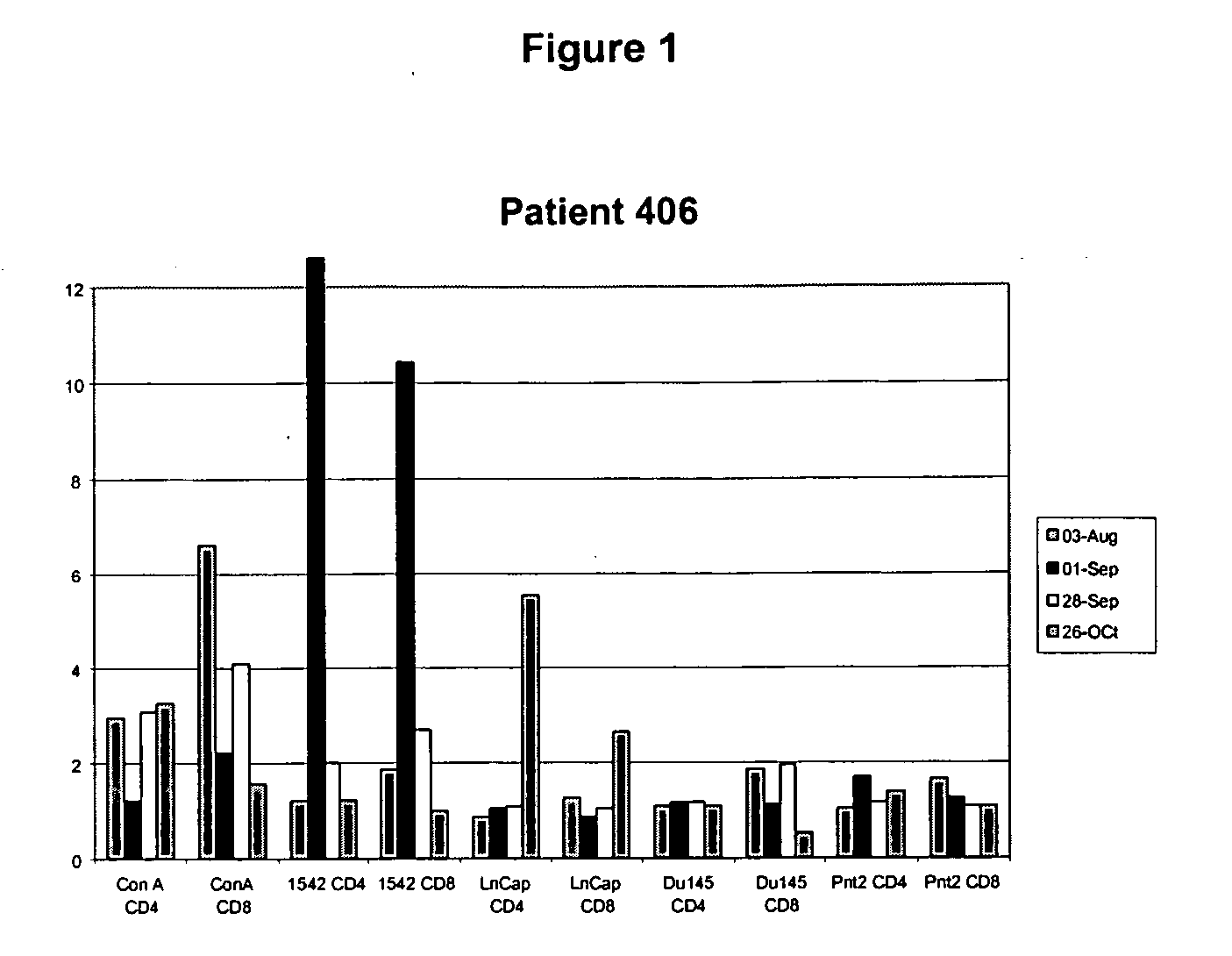
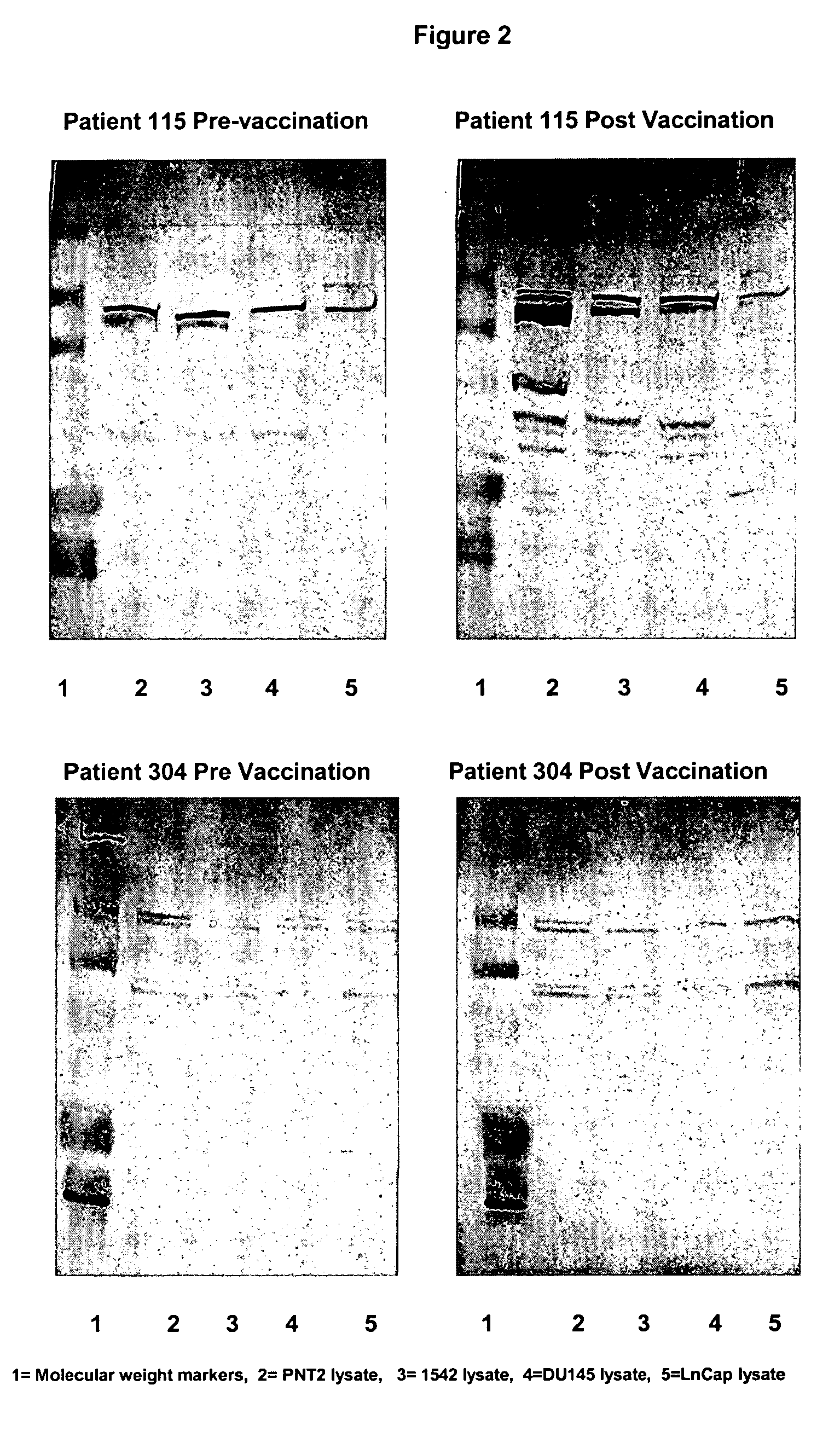
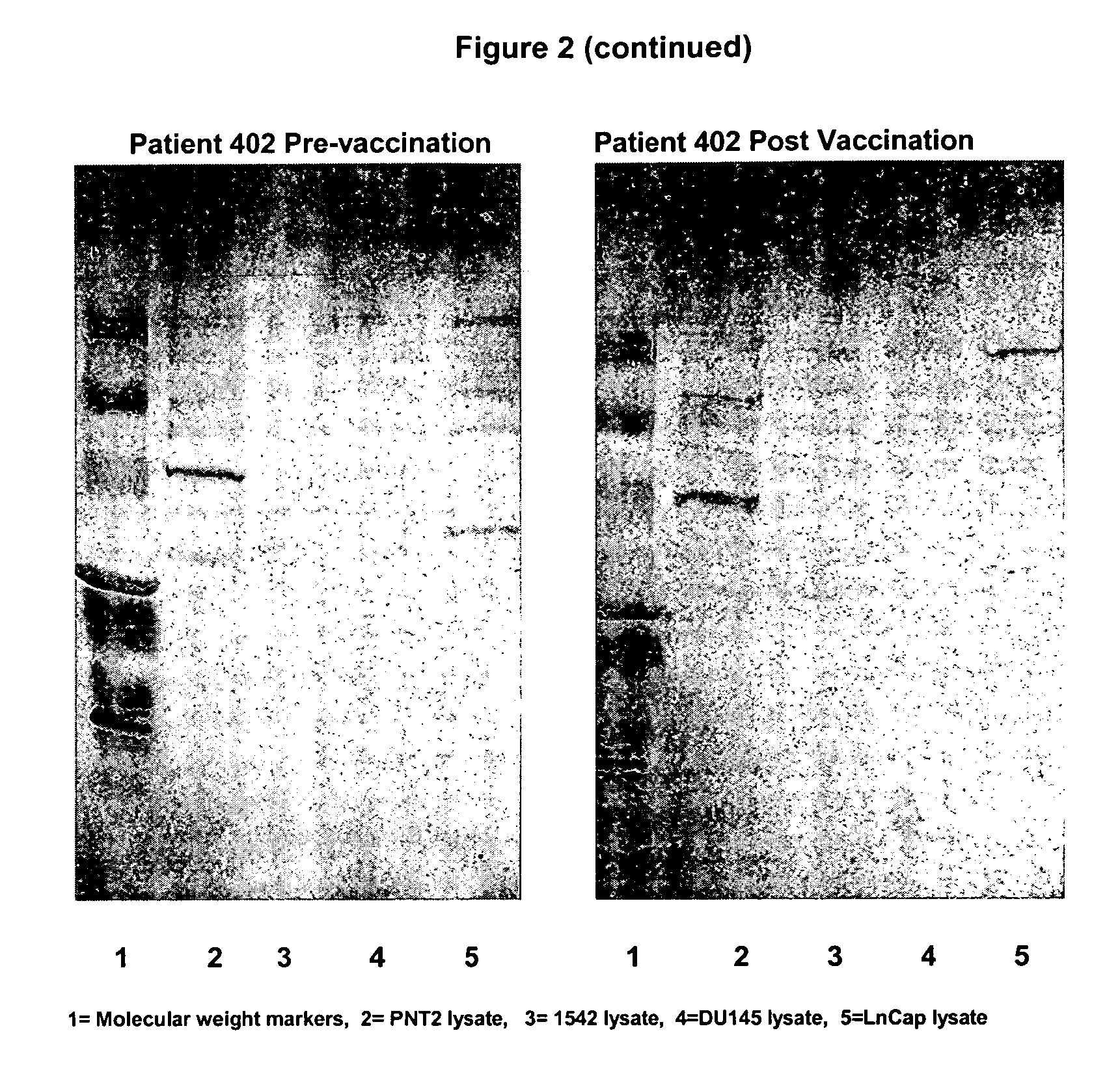
Human prostate cell lines in cancer treatment
a human prostate and cell line technology, applied in the field of human prostate cell lines in cancer treatment, can solve the problems of lack of targeting capability, inability to meet the expectations of patients, and inability to achieve specific immunomodulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Growth, Irradiation, Formulation and Storage of Cells
An immortalised cell line derived from normal prostate tissue namely PNT2 was grown in roller bottle culture in RPMI 1640 media supplemented with 2 mM L-glutamine and 5% fetal calf serum (FCS) following recovery from liquid nitrogen stocks. Following expansion in T175 static flasks the cells were seeded into roller bottles with a growth surface area of 850 cm2 at 1-20×107 cells per roller bottle.
An immortalized cell line derived from primary prostate tissue namely NIH1542-CP3TX was grown in roller bottle culture in KSFM media supplemented with 25μg / ml bovine pituitary extract, 5 ng / ml of epidermal growth factor, 2 mM L-glutamine, 10 mM HEPES buffer and 5% fetal calf serum (FCS) (hereinafter called “modified KSFM”) following recovery from liquid nitrogen stocks. Following expansion in T175 static flasks the cells were seeded into roller bottles with a growth surface area of 1,700 cm2 at 2-5×107 cells per roller bottle.
Two se...
example 2
Use of a Normal Melanocyte in a Murine Melanoma Protection Model Model
A normal melanocyte cell line was used in a vaccination protection model of murine melanoma utilising the B16.F10 as the challenge dose. The C57 mice received two vaccinations of either PBS, 5×106 irradiated K1735 allogeneic melanoma cells or 5×106 irradiated Melan Pi autologous normal melanocyte cells on days -14 and -7. Challenge on day 0 was with 1×104 B16.F10 cells and tumor volume measured every three days from day 10 onwards. Animals were sacrificed when the tumor had grown to 1.5×1.5 cm measured across the maximum dimensions of the tumor. It was found that that vaccination with Melan1P cells offer some level of protection against this particularly aggressive murine tumour, as seen in FIG. 5.
example 3
Phase I / II Study
A phase I / II study was carried out with three types of allogeneic cells representing a normal prostate cell line, a prostate tumour derived cell line and a metastasised tumour cell line. A combined cell vaccine was given to patients having hormone refractory prostate cancer and safety, tolerability and efficacy, as measured by effect on survival and quality of life determined. The following criteria were used for inclusion of patients: patients of any age with histologically confirmed prostate cancer; patients with hormonal refractory disease following optimal first line LHRH treatment, or high dose bicalutamide (150 mg per day), or orchidectomy; progressive disease indicated by a rise in serum PSA on at least 2 successive occasions separated by at least 4 weeks; serum PSA level of at least 2 ng / ml at Week -2; WHO Performance Status of 0-2 at Week -4; a life expectancy of at least 6 months; the ability of the patient to read and understand the patient information ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface area | aaaaa | aaaaa |
| surface area | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com