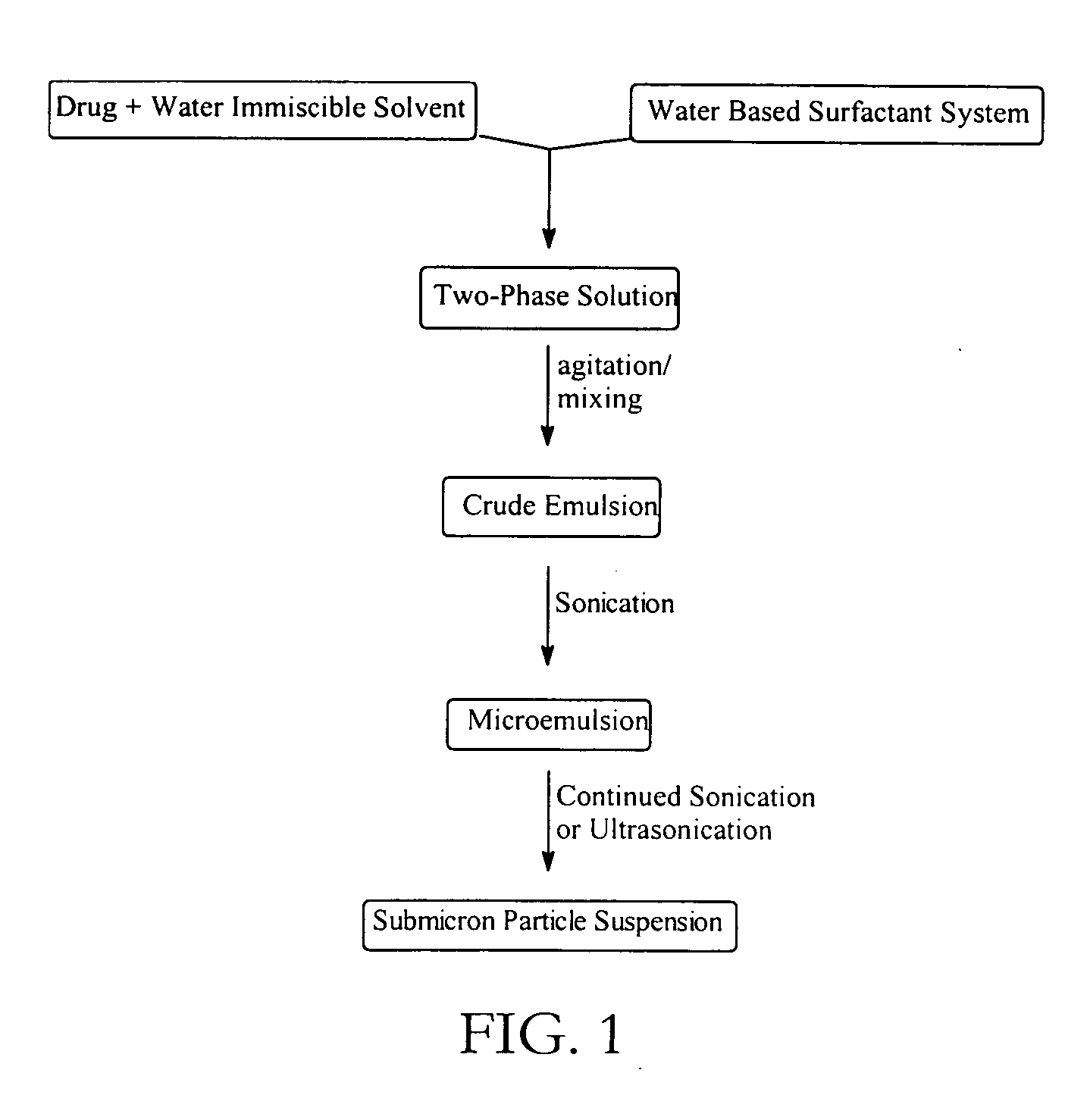
Preparation of submicron solid particle suspensions by sonication of multiphase systems
a multi-phase system and solid particle technology, applied in the direction of microcapsules, macromolecular non-active ingredients, chemical/physical processes, etc., can solve the problems of spontaneous formation of microencapsulated products, irritation at the site of delivery, and unstable emulsions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of a 0.5% Itraconazole Suspension Using a 1:10 Ratio of O / W
[0042] A 5% lecithin / glycocholate surfactant solution was prepared (100 mL) and combined with 10 mL of a chloroform solution containing itraconazole (0.5 grams). The resulting mixture was manually shaken to generate a crude emulsion and set in an ice bath to chill. After cooling for 5 minutes the emulsion was sonicated every other minute for 10 minutes (5 minutes total sonication time at 40% power using a ½″ probe at 20 kHz) and then rotovapped at ˜120 Torr (no heat) to remove the chloroform. The resulting solid particle dispersion was analyzed by light scattering detection (HORIBA) which revealed particles having a mean diameter of 97.78 nm.
example 2
Preparation of a 1.0% Itraconazole Suspension Using a 1:5 Ratio of O / W
[0043] A 5% lecithin / glycocholate surfactant solution was prepared (50 mL) and combined with 5 mL of a chloroform solution containing itraconazole (0.5 grams). The resulting mixture was manually shaken to generate a crude emulsion and set in an ice bath to chill. After cooling for 5 minutes the emulsion was sonicated every other minute for 10 minutes (5 minutes total sonication time) and then rotovapped at ˜100 Torr (no heat) to remove the chloroform. The resulting solid particle dispersion was analyzed by light scattering detection (HORIBA) which revealed particles having a mean diameter of 135 nm.
example 3
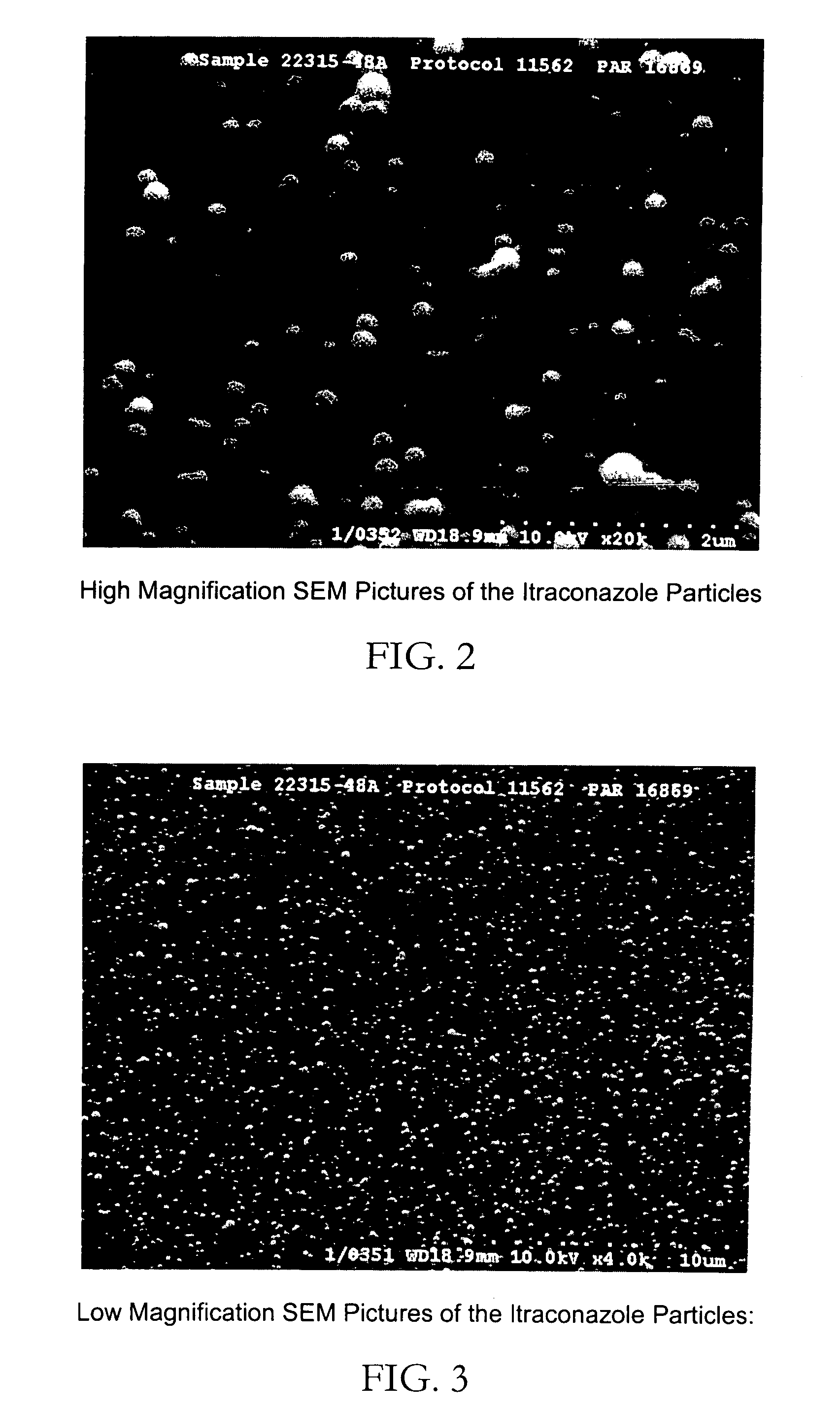
[0044] The process described in example 1 was repeated with the resulting particles having a mean diameter of 139 nm. This suspension was further analyzed by scanning electron microscopy to reveal solid spherical particles less than 200 nm in size. See FIG. 2.
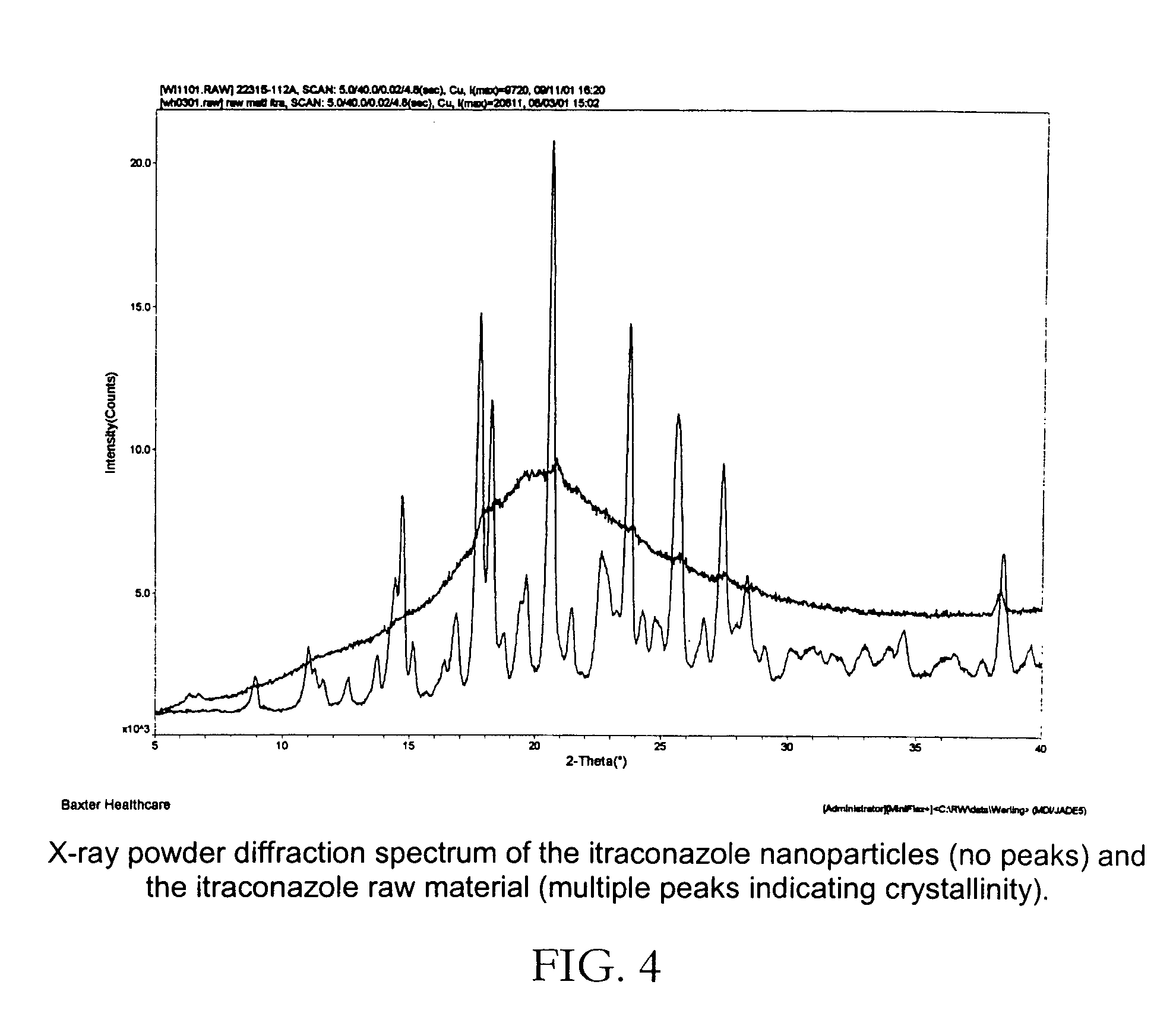
[0045]FIG. 2 reveals the spherical nature of the particles produced. The sample was prepared by filtration of a small portion o the suspension through a 80 nm filter and using standard SEM sample preparation techniques. Analysis of particles produced by this process revealed the particles to be completely amorphous as determined by x-ray powder diffraction.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com