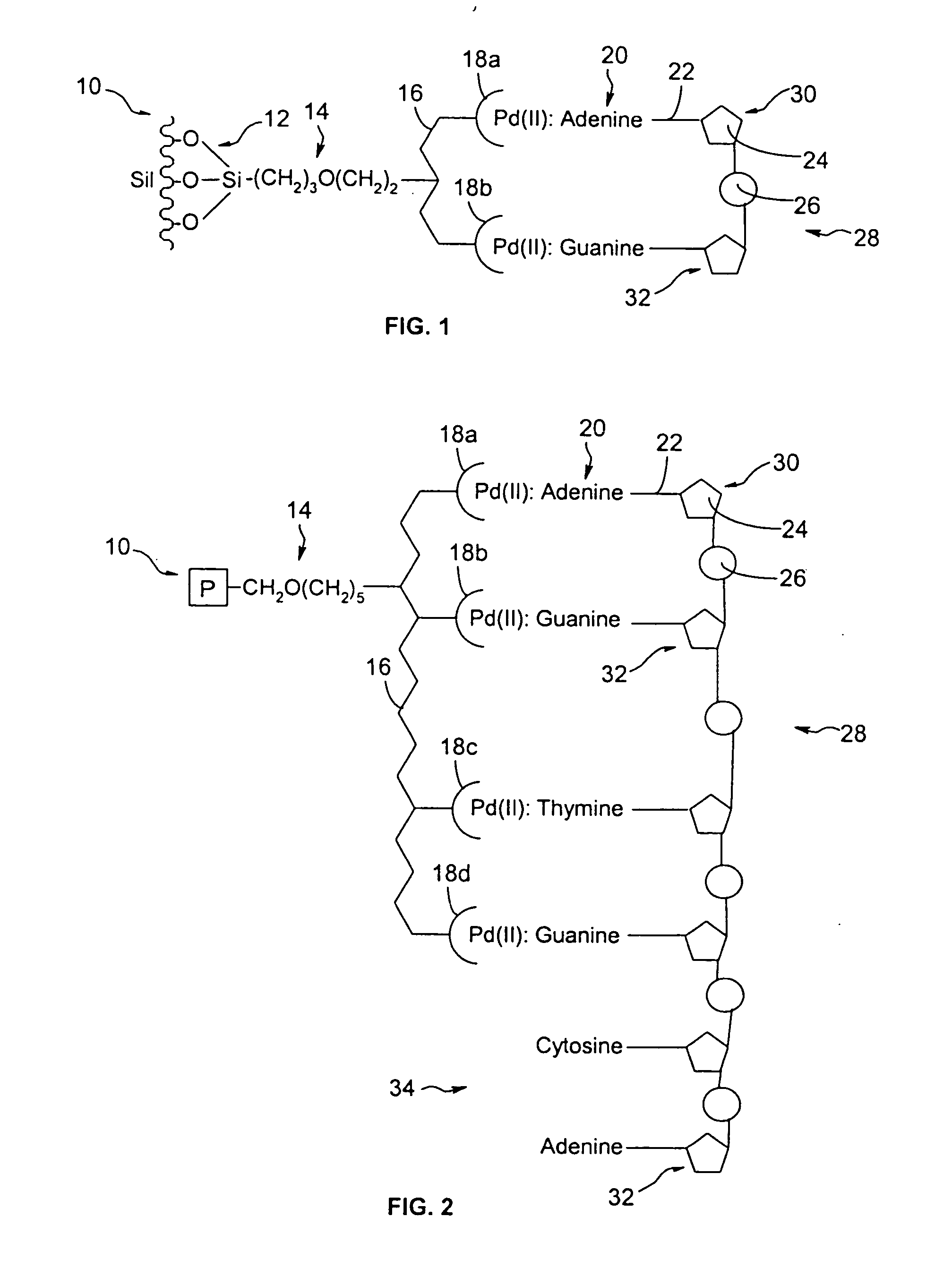
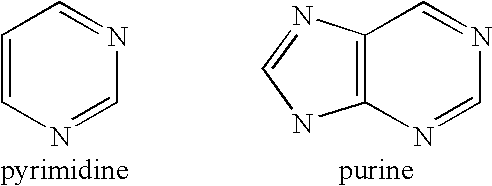
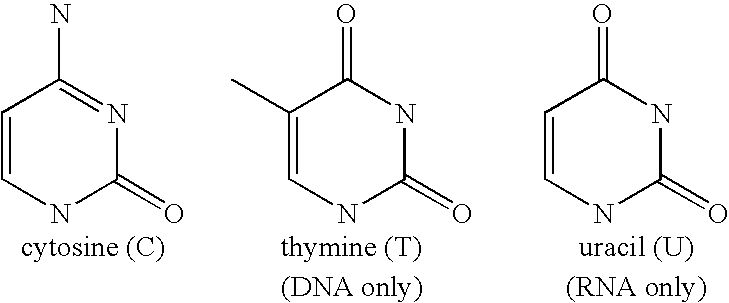
Compositions and methods for separating heterocyclic aromatic amine bases, nucleosides, nucleotides, and nucleotide sequences
a technology of aromatic amine bases and which is applied in the preparation of sugar derivatives, group 1/11 element organic compounds, sugar derivatives, etc., can solve the problems of low selectivity of systems and methods, and the inability to separate lone heterocyclic aromatic amine bases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Diethylenetriamine Bonded to Silica Gel
To 0.3 L of toluene was added 13 g of γ-glycidoxypropyltrimethoxysilane and 6.24 g of diethylenetriamine and the mixture was stirred overnight. To the mixture was added 60 g of silica gel (35-60 mesh) and the product was heated at 80° C. for 16 hours. A product was filtered off, washed with water and methanol, and dried under reduced pressure. The product produced can have one of the following structure (a or b):
example 2
Preparation of Polystyrene Bonded to Diethylenetriamine
About 0.5 g of chloromethylpolystyrene (predried by coevaporation with 50 ml of benzene) was heated at 120° C. with 10 g of diethylenetriamine for 24 hours. After the product was cooled, the beads were collected by filtration, washed with CH3OH, and dried under reduced pressure. The product produced can have one of the following structure (a or b):
example 3
Preparation of Tetramethyldiethylenetriamine Bonded to Silica Gel
To a well-stirred solution of 1 g tetramethyldiethylenetriamine and 0.8 ml of triethylamine in 75 ml DMF at room temperature was added 1.0 ml of bromopropyltrimethoxysilane dropwise. The resulting mixture was heated to 80° C. After 16 hours, 11 g of silica gel was added. After an additional 4 hours, the reaction product was cooled to room temperature. The solution was decanted and silica gel was added to the THF, filtered, and washed with excess THF and methanol. Next, the product was dried under vacuum for 16 hours at 55° C., producing about 11.5 g of tetramethyldiethylenetriamine bonded to silica gel. The reaction formula is shown below:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com