A kind of monocyclic benzoxazine intermediate and its preparation method and application
A technology of benzoxazine and intermediates, which is applied in the preparation and application of high-performance resin intermediates, can solve problems such as limitations and complex synthesis processes, and achieve high heat resistance, high cross-linking density, and high degree of carbonization Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
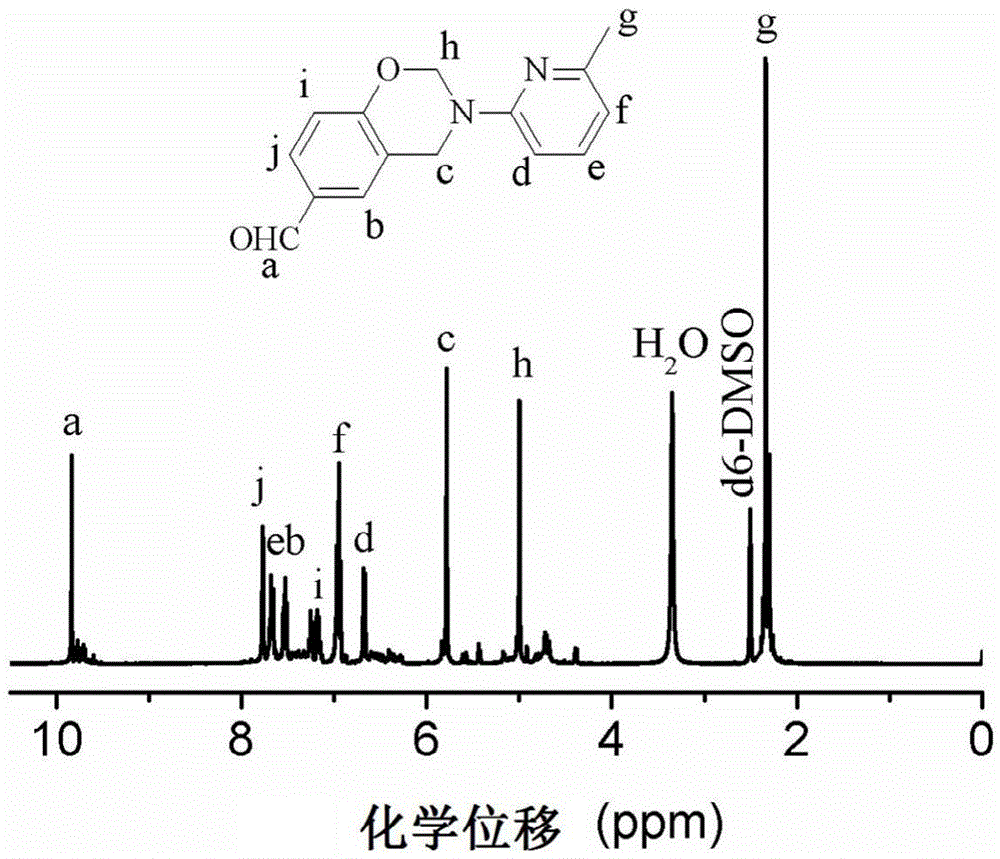
[0047] Add 0.2mol of 2-amino-6-picoline, 0.40mol of formaldehyde and 40mL of toluene into the reaction flask, and react for 0.1h; / L sodium hydroxide aqueous solution was washed once and then washed twice with deionized water, and the solvent was removed by rotary evaporation under reduced pressure, and dried, with a yield of 89%.
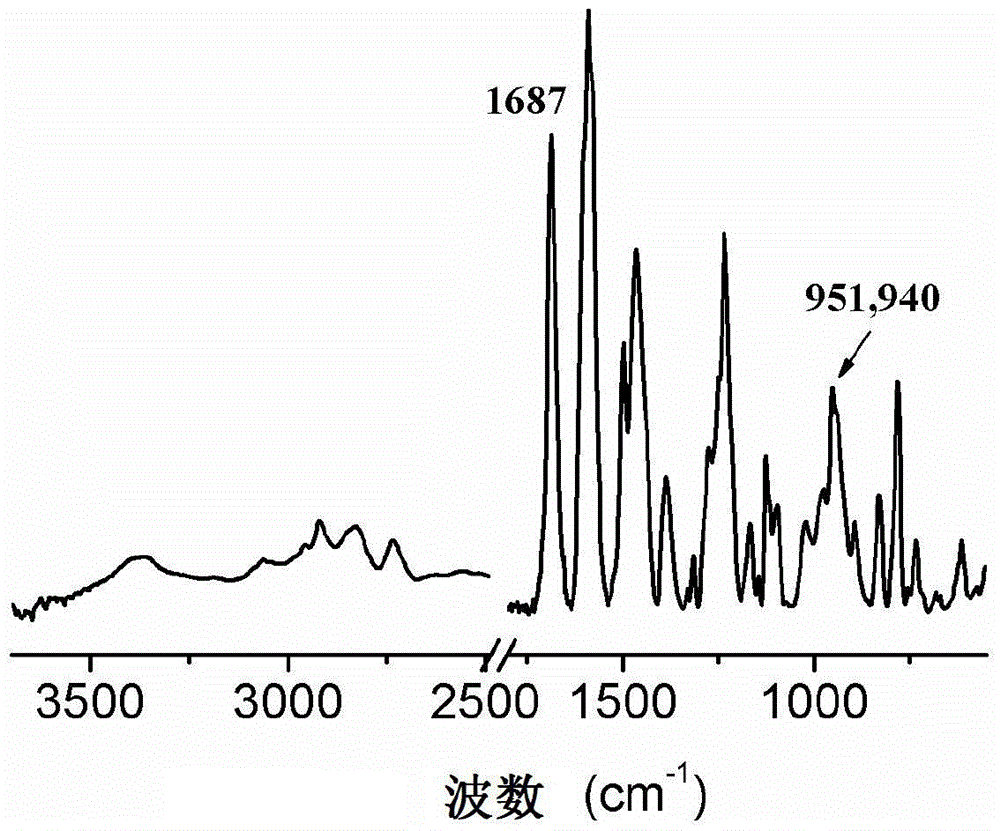
[0048] FTIR (KBr, cm -1 ): 951,940 (characteristic peak of oxazine ring), 1386 (-CH 3 ), 1687 (-CHO).
Embodiment 2
[0050] Add 0.2 mol of 2-amino-6-picoline, 0.41 mol of formaldehyde and 40 mL of ethanol into the reaction kettle, and react for 0.5 h; L of sodium hydroxide aqueous solution was washed once and then washed four times with deionized water, the solvent was removed by rotary evaporation, and dried. The yield was 84%.
[0051] FTIR (KBr, cm -1 ): 948 (characteristic peak of oxazine ring), 1380 (-CH 3 ), 1663 (-CHO).
Embodiment 3
[0053] Add 0.2 mol of 2-amino-4-methylpyrimidine, 0.43 mol of formaldehyde and 40 mL of dioxane into the reaction kettle, react for 0.8 h; Wash once with 2 mol / L sodium hydroxide aqueous solution, then wash three times with deionized water, remove the solvent by rotary evaporation, and dry. The yield is 89%.
[0054] FTIR (KBr, cm -1 ): 956 (characteristic peak of oxazine ring), 1383 (-CH 3 ), 1687 (-CHO).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com