Detergent formulations containing alkaline peroxide salts and organic acids
a technology of alkaline peroxide salts and formulations, applied in the field of cleaning compositions, can solve the problems of inability to clean colored or figured fabrics, agents with peculiar smells, and limited cleaning of compatible fabrics, and achieve the effect of increasing storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
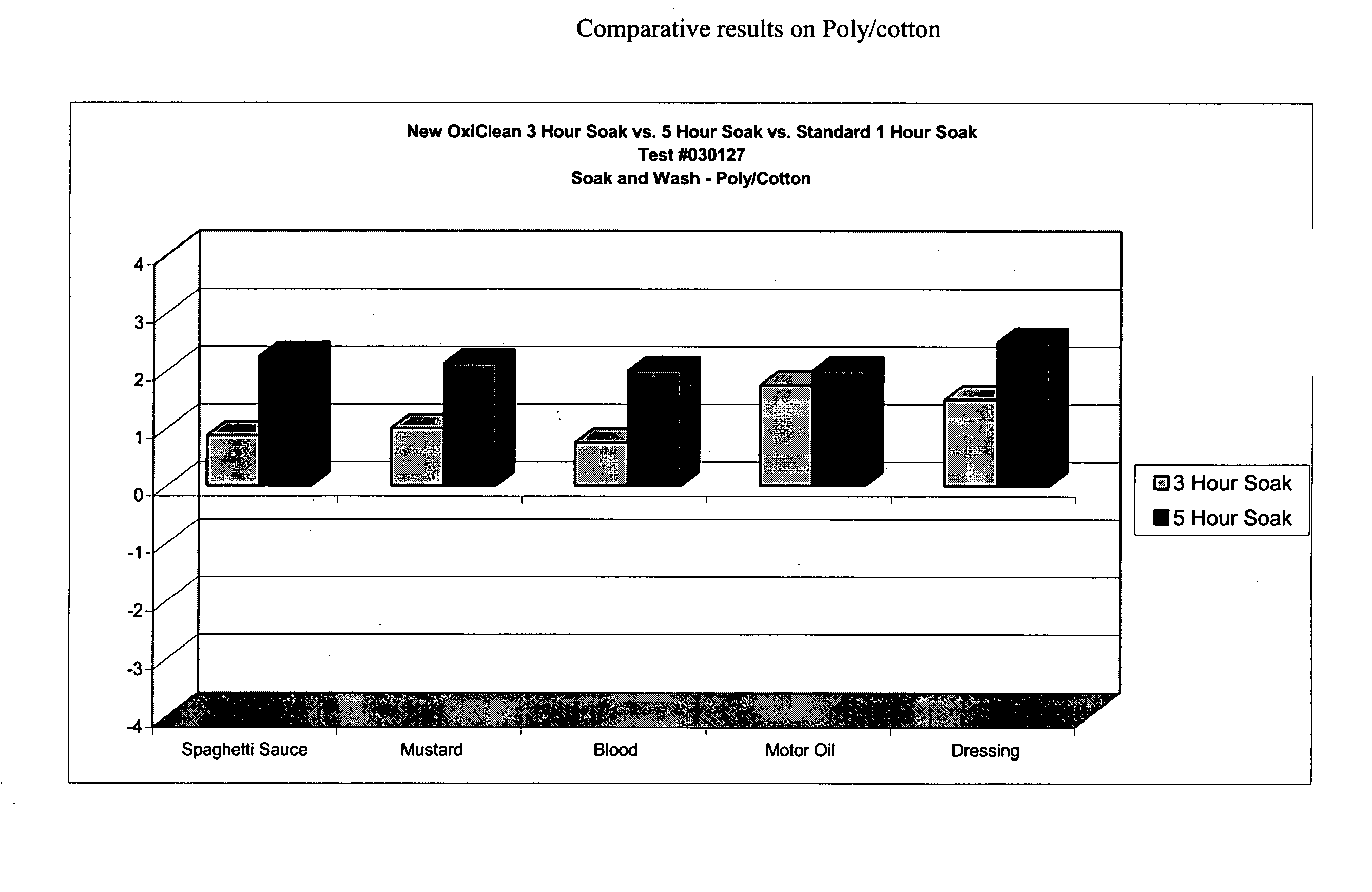
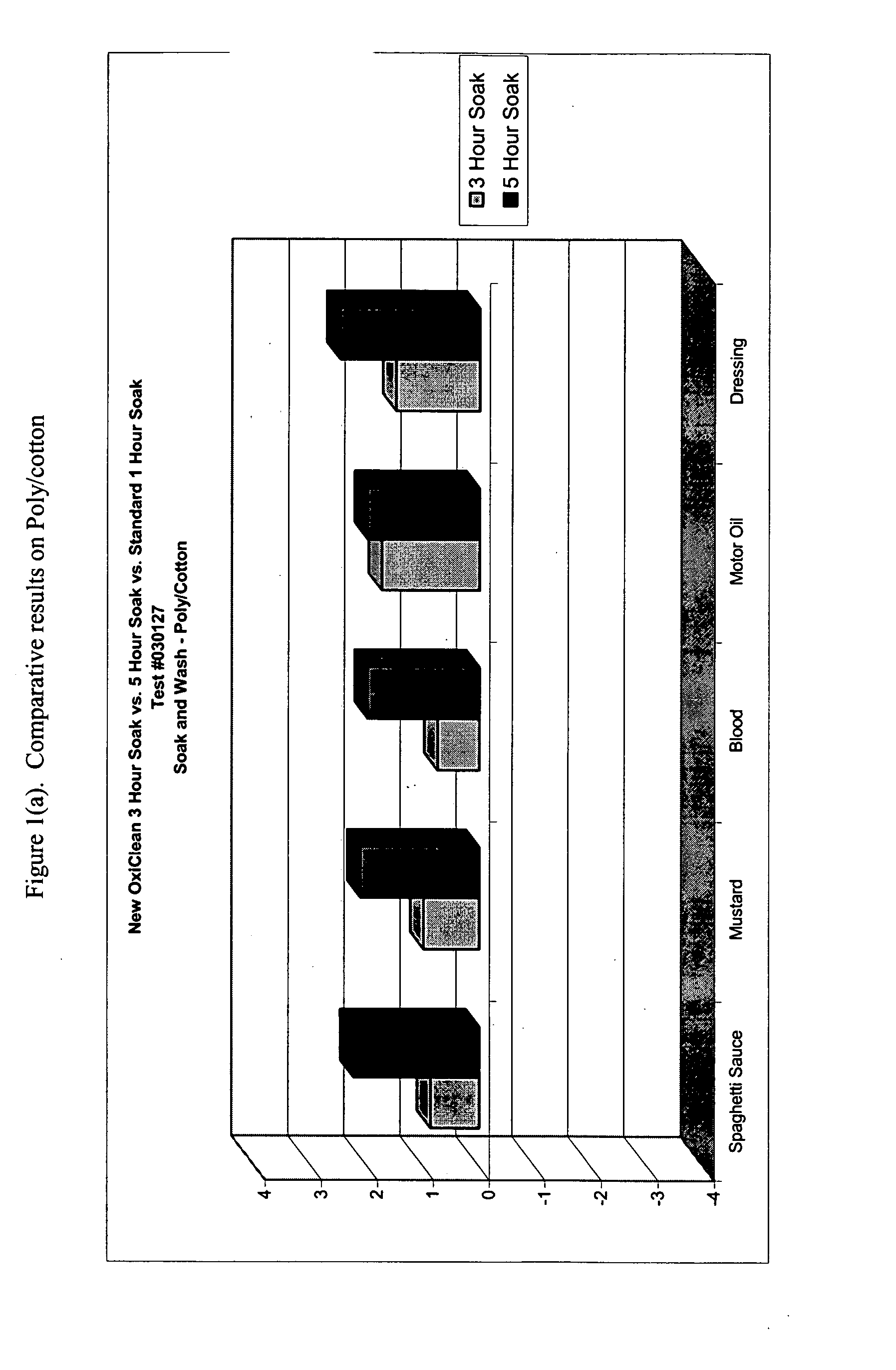
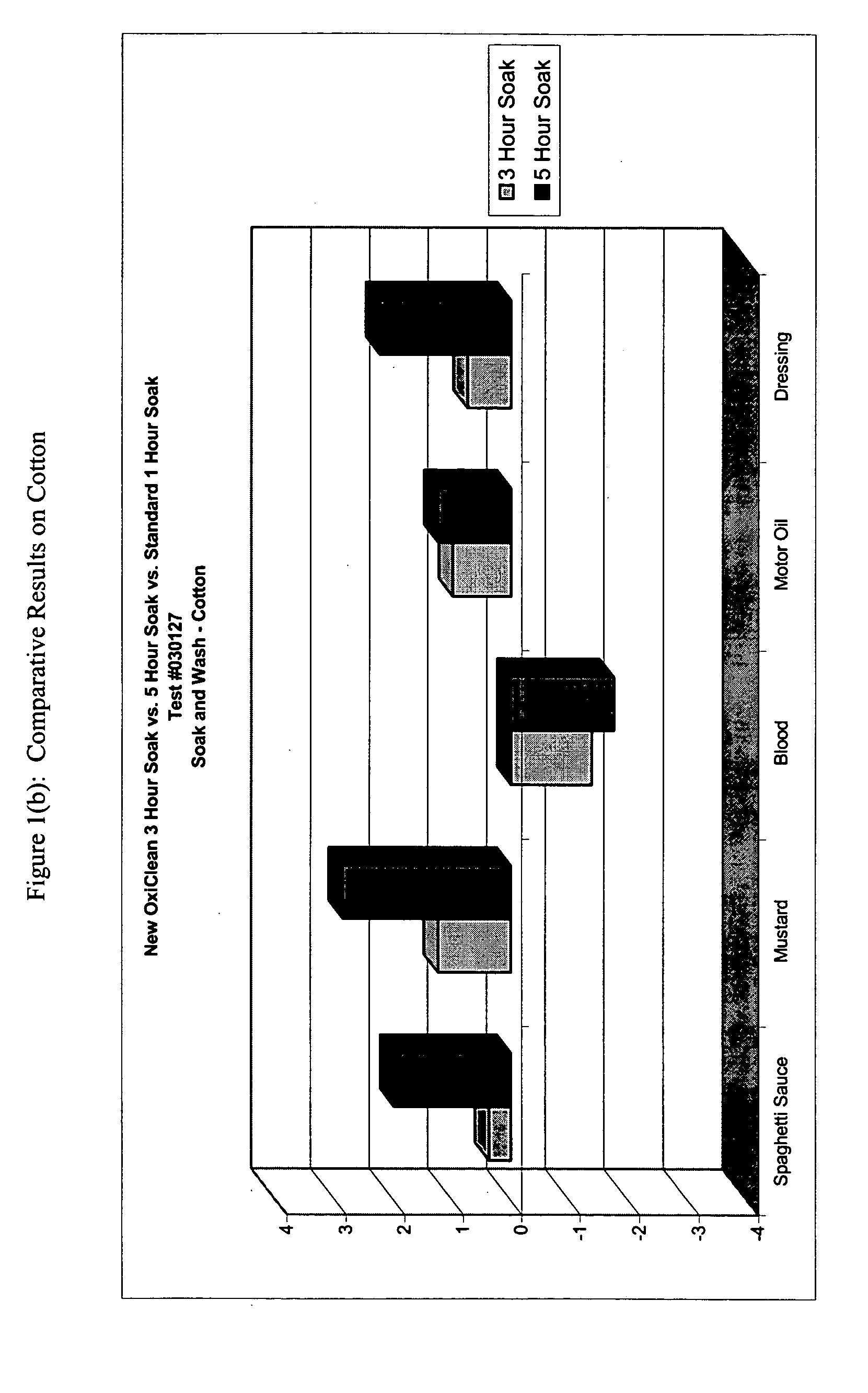
This example demonstrates the improved efficacy of the detergent composition described in Table 1 (hereinafter denoted as the “Improved Formulation”) in the removal of various types of stains from cotton, poly / cotton and cotton knit fabrics during a one-, three- or five-hour pre-soak.
Six-inch square swatches of prewashed, cut cotton cloth, polyester / cotton cloth, and cotton knit cloth were obtained from Testfabrics, Inc. (West Pittston, Pa.) Five different stains were tested: spaghetti, mustard, salad dressing, dirty motor oil, and blood. For each time point, duplicate swatches were used.
Stains were applied in the following manner. For spaghetti, 0.25 mL of RAGU™ Original was applied to each fabric swatch. For mustard, KROGER™ Traditional Yellow was applied to each fabric swatch by placing a small quantity of the mustard to the back of a rubber pipette bulb, then rubbing the mustard onto the fabric swatch. To approximate salad dressing stains, 0.25 mL KRAFT FREE™ Catalina dress...
example 2
This example compares the stain removal efficacy of two detergent formulations of the present invention containing different peracid precursors, with a formulation that does not contain peracid precursors that is described in Table 4 (hereinafter the “Comparison Formulation”).
TABLE 4Components of the Comparison FormulationComponentWt. %CAS #NameSurfactant38.17 497-19-8Sodium carbonatesubstrateNon-ionic2.95 68551-12-2GENOPOL LA060 ™; linearsurfactantC12-C16 alcohol ethoxylate (6moles EO); Clariant CorporationNon-ionic0.65127036-24-2GENOPOL UD-030 ™; C-11surfactantalcohol ethoxylate (3 moles EO);Clariant CorporationWater0.40 34590-94-8DPM; Dipropylene glycol methylsolubleether (1-(2-methoxyisopropoxy)-solvent2-propanol); LyonellAlkaline56.58 15630-89-4Sodium percarbonate; Solvay-peroxide saltInteroxDispersive0.25 68479-09-4acrylic acid copolymer ˜4500agentmole wt., sodium neutralized;Rohm and HaasMetals1.00 6834-92-0Blue Meta; Sodium Metasilicate,protectant / anhydrous 100.00; PQdis...
example 3
This example compares the stain removal performance of the Improved Formulation, the Comparison Formulation and several commercially available laundry pretreatments.
Six inch square swatches of prewashed, cut cotton cloth and polyester / cotton cloth were obtained from Testfabrics, Inc. Nine different stains were tested: coffee, grape, spaghetti, blood, dressing, grass, makeup, mustard, and pie filling. Each stain was applied to duplicate swatches of each fabric for each pretreatment type. To apply the stains, the following staining procedures were used. Starting materials for each stain were those generally used in the laundry additive arts. The starting materials for grass and pie filling were homogenized. All stains were applied in an amount sufficient to create a saturated, 1-inch circle of stain on each fabric. Stained fabric swatches were allowed to age overnight, after which the fabric swatches were treated with the indicated treatments. Treatment conditions were as follows. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com