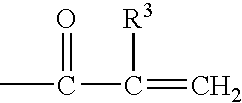
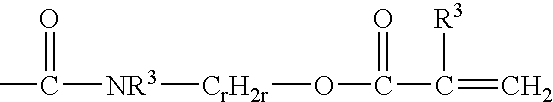
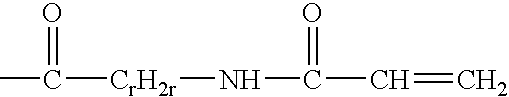Reactive hydrophilic oligomers
a technology of reactive hydrophilic oligomers and crosslinkable oligomers, which is applied in the direction of bandages, dermatological disorders, drug compositions, etc., can solve the problems of insufficient absorption and cohesive strength, and achieve the effects of high performance materials, minimal shrinkage, and purity of materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A hydrophilic copolymer with photoinitiator pendant groups was synthesized by charging an amber glass bottle with 99.0 g of MPEG, 1.0 g of DAROCUR ZLI-3331, 0.1 g of α-methyl styrene, 0.4 g of VAZO 52 and 100 g of ethyl acetate. The contents of the bottle were purged with N2 for 20 minutes and the bottle was capped, using poly(tetrafluoroethylene) thread tape (available from 3M Co., St. Paul, Minn.) between the cap and the threads of the bottle. The bottle was placed in a Model LLF LAUNDER-O-METER (available from Atlas Electric Devices Co., Chicago, Ill.), with the water bath temperature set at 60° C., for 16 hours. The resulting polymer had a Mn of 36,900 as determined by GPC.
example 2
A hydrophilic copolymer with photoinitiator pendant groups was synthesized by charging an amber glass bottle with 65.0 g of MPEG, 35.0 g of HEMA, 1.0 g of DAROCURE ZLI-3331, 4.0 g of α-methylstyrene, 0.4 g of AIBN and 90 g of ethyl acetate. The contents of the bottle were purged with nitrogen gas for 20 minutes and the bottle was capped, using poly(tetrafluoroethylene) thread tape. The bottle was placed in a water bath shaker at 65° C. for 24 hours. The resulting polymer had a Mn of 15,700 as determined by GPC.
example 3-7
A series of hydrophilic copolymers were synthesized by charging 5 amber glass bottles with different weight proportions of hydrophilic monomers and a-methyl styrene as shown in Table 1. Sufficient ethyl acetate was added to each bottle so that the total monomer concentration was 50 weight percent. VAZO 52 (0.4 g) was added to each bottle. The contents of each bottle was purged with nitrogen gas for 20 minutes and the bottle was capped, using poly(tetrafluoroethylene) thread tape. Each bottle was placed in a Model LLF LAUNDER-O-METER, with the water bath temperature set at 60° C., for 16 hours. Compositions and Mn values for Examples 3-7 are shown in Table 1.
TABLE 1Examples 3-7MPEG-Alpha-ExampleMPEG(g)HEMA(g)DMACM(g)NVA(g)1000(g)methylstyrene(g)Mn393.74.91.043,000498.51.00.134,900564.14.929.61.037,100664.14.929.61.023,80071.098.50.113,200
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com