Assay for evaluating the therapeutic effectiveness of agents in reducing Alzheimer's disease pathology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
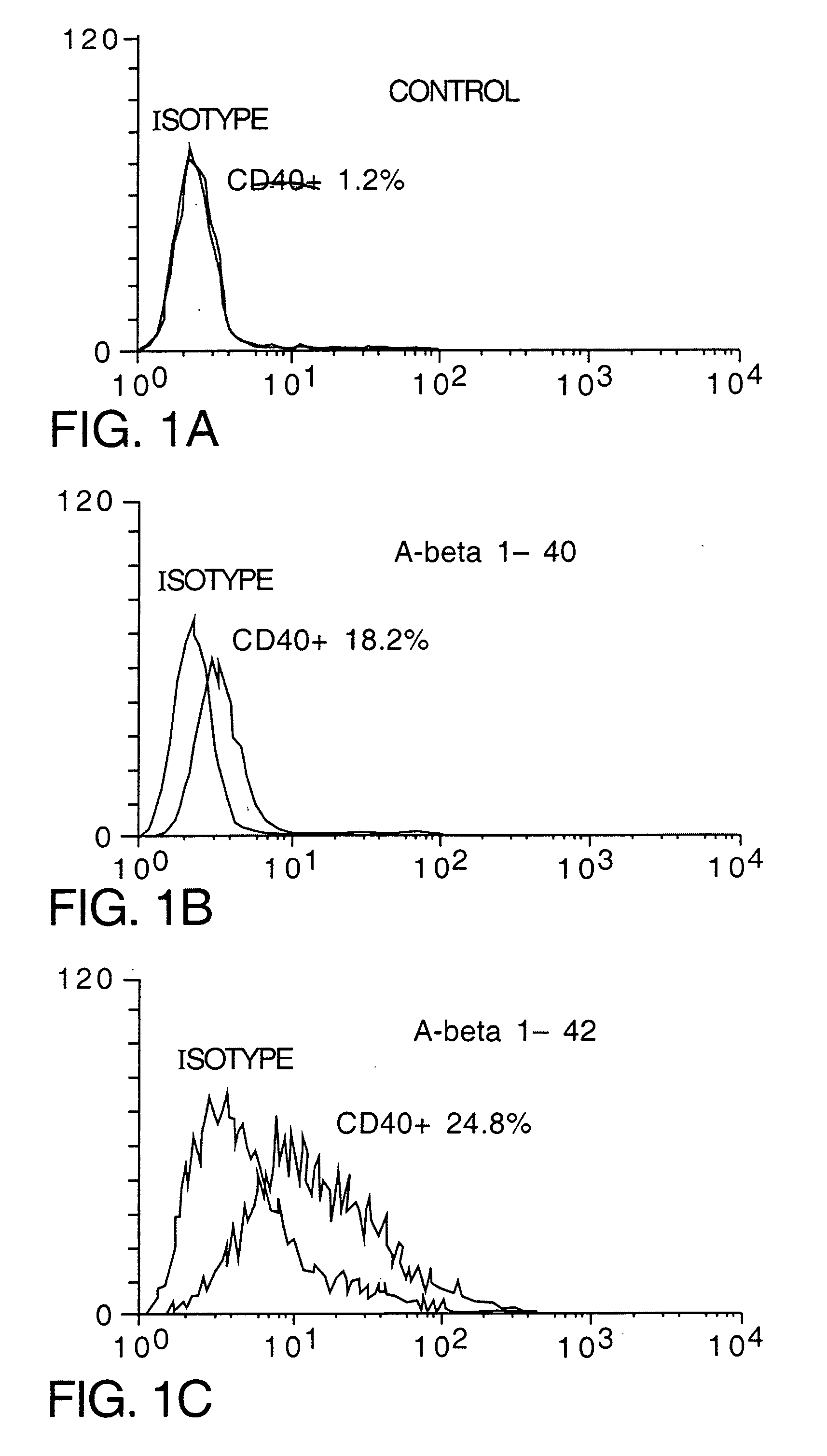
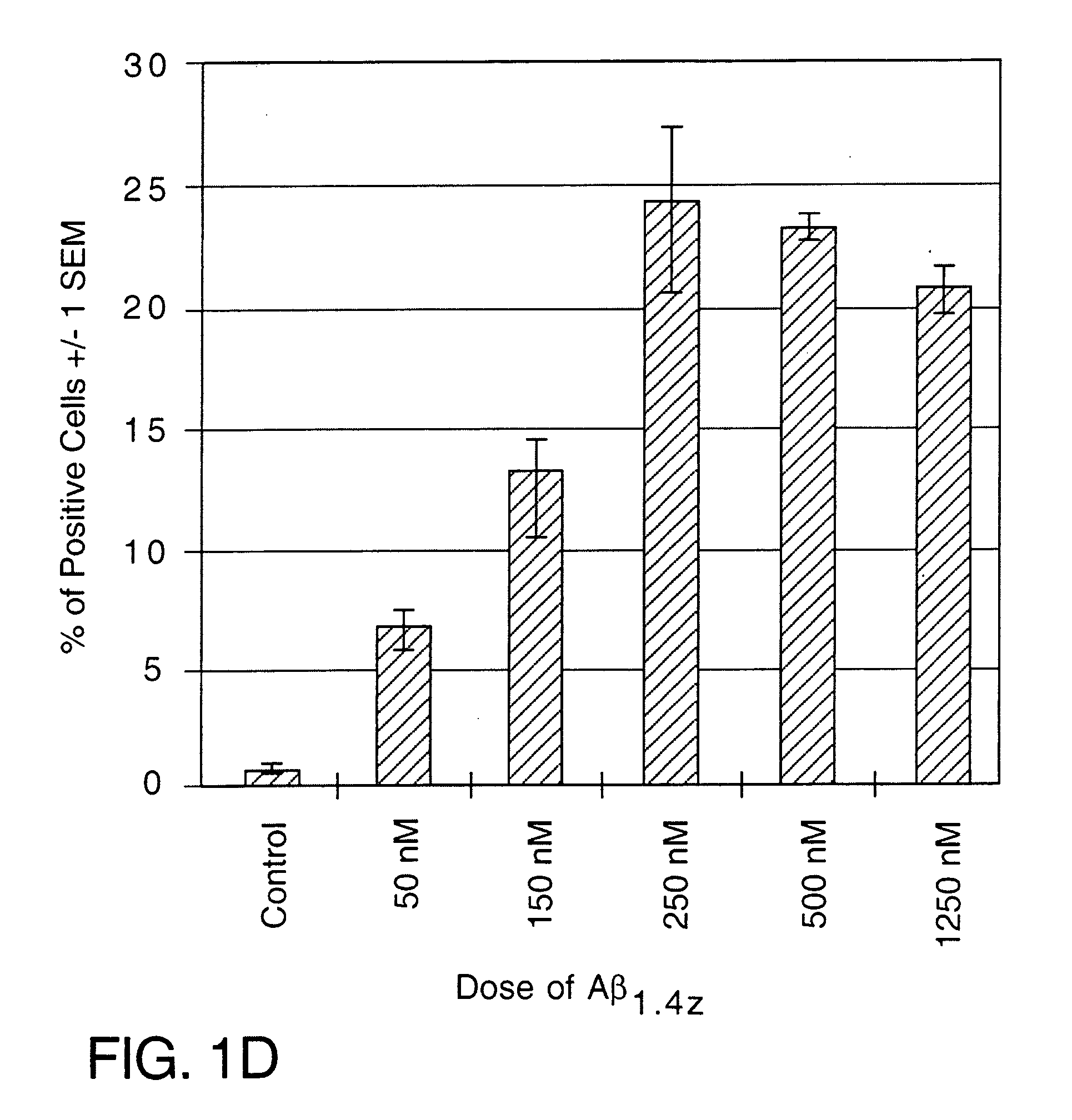
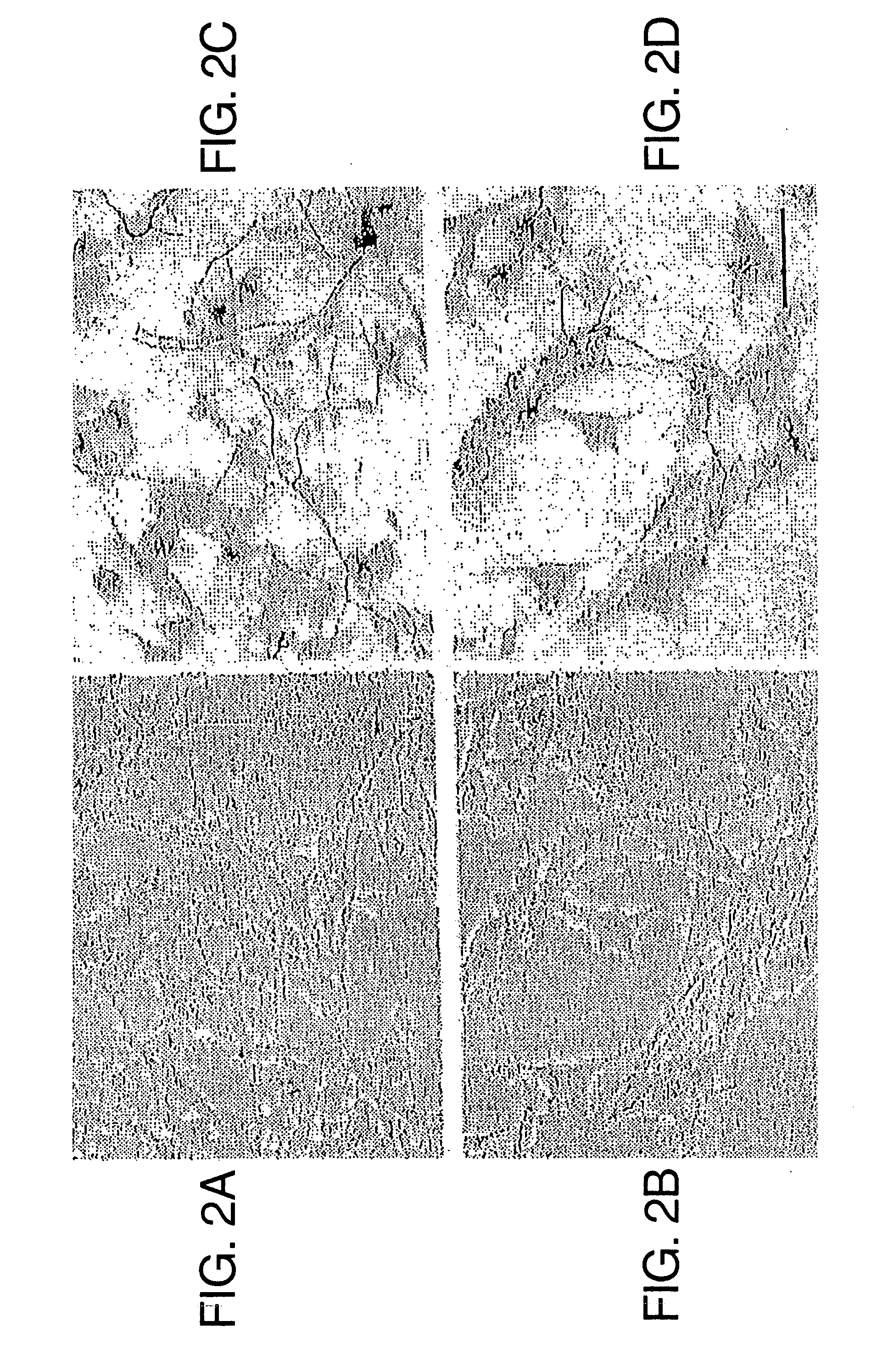
[0079] To determine whether Aβ could induce CD40 expression in cultured N9 and N60 microglial cells, cells were treated with 250 nM of freshly solublized Aβ1-40 or Aβ1-42. As shown in both FIG. 2A, both Aβ1-40 and Aβ1-42 significantly induce CD40 expression on cultured microglial cells when compared to Aβ-free, reverse Aβ (Aβ40-1) or the 695 isoform of soluble amyloid precursor protein (sAPPα-695) when compared to control peptide (human thyrocalcitonin) or Aβ free conditions. In addition, Aβ1-42 induced microglial CD40 expression in a dose dependent manner (from 50 to 1250 nM, FIG. 2B).
[0080] To determine whether endogenous overexpression of Aβ could lead to microglial CD40 expression, CD40 expression on microglia was measured from a transgenic mouse model of AD (Tg APPSW, overexpressing Aβ1-40 and Aβ1-42), (11) and control (wild-type) littermates (12). Microglia from Tg APPSW newborn mice had markedly increased levels of soluble Aβ1-40 compared to control littermates (13), and the...
example 2
Materials and Methods
Endothelial Cell Culture and Reagents
[0088] HAEC and HAEC medium were purchased from Clonetics (San Diego, Calif.). HAEC were cultured and expression assays were performed as previously described [29]. Aβ1-40, Aβ1-42, and reverse Aβ40-1, (control) peptides were obtained from QCB (Hopkinton, Mass.). Reverse transcriptase polymerase chain reaction (RT-PCR) kits and RNA reagents were obtained from Invitrogen Inc. (San Diego, Calif.). Human IFN-γ recombinant protein was purchased from Genzyme (Cambridge, Mass.).
RT-PCR
[0089] Cultured HAEC were plated at 1×105 cells / well in 6-well culture plates (Falcon, Becton Dickinson Inc. N.J.). HAEC were treated with freshly solubilized Aβ1-40, or Aβ1-42, (500 nM, in dH20), control peptide (500 nM) or IFN-γ (10 U / mL) for 48 hours after plating. Total RNA was isolated, and cDNA was prepared as previously described [30]. PCR was performed for 30 cycles, with each cycle consisting of 94° C. for one minute, 55° C. for two minut...
example 3
Materials and Methods
Materials
[0102] Cell culture media, fetal bovine serum (FBS) and other culture reagents were supplied by Clonetics, GibcoBRL and Sigma. Aβ1-40, Aβ1-42 and Aβ25-35 were supplied by RBI and / or M.D. Enterprise. All Aβ peptides used were freshly dissolved in Sigma H2O and aliquots were promptly stored at −20° C. The ABC-based enzyme-linked immunoassay (ELISA) kit was obtained from Sigma. The monoclonal antibodies (mAbs) against human CD40, CD45, CD40L, IFN-γR , IL-1β, IFN-γ ELISA kit was ordered from R&D Systems or Endogen, respectively.
Cell Cultures
[0103] Human aortic endothelial cell (HAEC, 3rd passage) line was obtained from Clonetics, and grown in endothelial cell growth medium (EGM, Clonetics) containing endothelial cell basal medium, supplemented with 10 ng / ml human recombinant epidermal growth factor, 1 μg / ml hydrocortisone, 12 μg / ml bovine brain extract, 2% FBS, 50 μg / ml Gentamicin and 50 ng / ml Amphotericin B. As described by the manufacturer, this HAE...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Therapeutic | aaaaa | aaaaa |
| Interaction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com