Quick disintegrating tablet in buccal cavity and production process thereof
a technology of disintegrating tablets and buccal cavity, which is applied in the direction of securing communication, digital transmission, star/tree networks, etc., can solve the problems of small strength, inability to take over the preparation of ptp, and some problems with the dosage form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
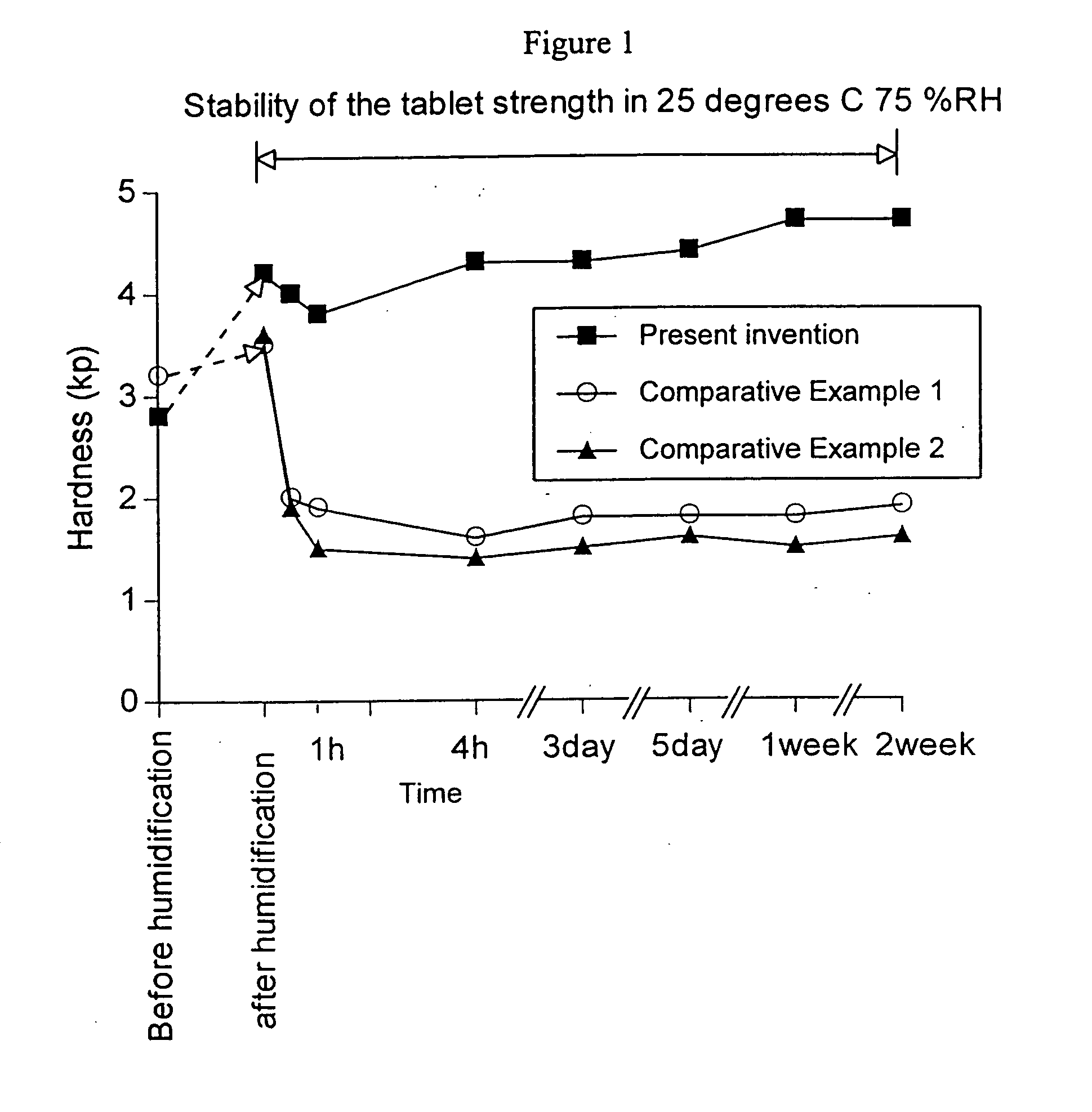
[0031] Mannitol 602 g and lactose 602 g were mixed. This was passed through a sieve (14 mesh). 433 g of glucose solution (15 w / v %) was used as a binding agent for this mixture, and the mixture was granulated in a fluidized-bed granulator. Up to 157 g of the solution was used to coat the above mixture at a spray pressure of 2.5 kg / cm2. Afterwards, it was granulated with spray pressure 1.5 kg / cm2. After drying the granule, peppermint flavor 10g, stearic acid 12g, magnesium stearate 10g were combined. Rotary tablet machine was used to manufacture tablets which were 540 mg per one tablet (tablet hardness 1.4 kp (n=5)). Next, this tablet was humidified and heated for 20 minutes in a thermo-hygrostat at 35° C., 85% RH. Afterwards, it was dried for 15 minutes at 50° C. (humidity 30%), and the tablet of the present invention was achieved. The obtained tablet had hardness of 9.1 kp, and buccal cavity disintegrating time of 17 seconds.
example 2
[0032] 175 g of a lactose solution (10 w / v %) was a binding agent for 350 g of lactose (Domo milk Corp.). This was granulated in a fluidized-bed granulator (Ohkawara Seisakusho). Up to 70 g of the previous solution was used to coat the lactose with a spray pressure of 2.5 kg / cm2. Afterwards, it was granulated with a spray pressure 1 kg / cm2. After drying the granule, 0.5% magnesium stearate was mixed with the granule. Tablets ((phi 10 mm, 10 mmR), tablet hardness 2.3 kp (n=5)) of 300 mg per tablet were produced using a rotary tablet machine. Next, the tablet was stored under heated humidified conditions of 25° C. / 70% RH for 19 hours, using a thermo-hygrostat (Tabiespec Corp., PR-35C). Afterwards it was dried for 2 hours at 25° C. (humidity 50%). The tablet of the present invention was obtained. The obtained tablet had a hardness of 4.1 kp (n=5) and a buccal cavity disintegration time of 20 seconds.
example 3
[0033] 378 g of mannitol (Towa Chemical Industry Corp.) was passed through a sieve (20 mesh). Afterwards, this was granulated in a fluidized bed granulator (Ohkawara Seisakusho) with 133 g of an aqueous solution of hydrated crystalline glucose (Nippon Shokuhin Kako Corp.) (15 w / v %) as a binding agent. Up to 50 g of the previous solution was used to coat the mannitol with a spray pressure of 2.5 kg / cm2. Afterwards, it was granulated with a spray pressure 1.5 kg / cm2. At this time, the disappearance of the absorption peak derived from glucose crystals (i.e. glucose is amorphous) was confirmed using a differential scanning calorimeter (DSC for short). 0.5% magnesium stearate was mixed with the granule. Tablets ((phi 8 mm, 9.6 mmR), tablet hardness 2.0 kp (n=5)) of 150 mg per tablet were produced using a rotary tablet machine with a compression pressure of approximately 0.18 ton / punch. Next, the tablet was stored under heated humidified conditions of 25° C. / 70% RH for 24 hours, using a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com