Hydrocracking catalyst and method of hydrocracking heavy oil
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
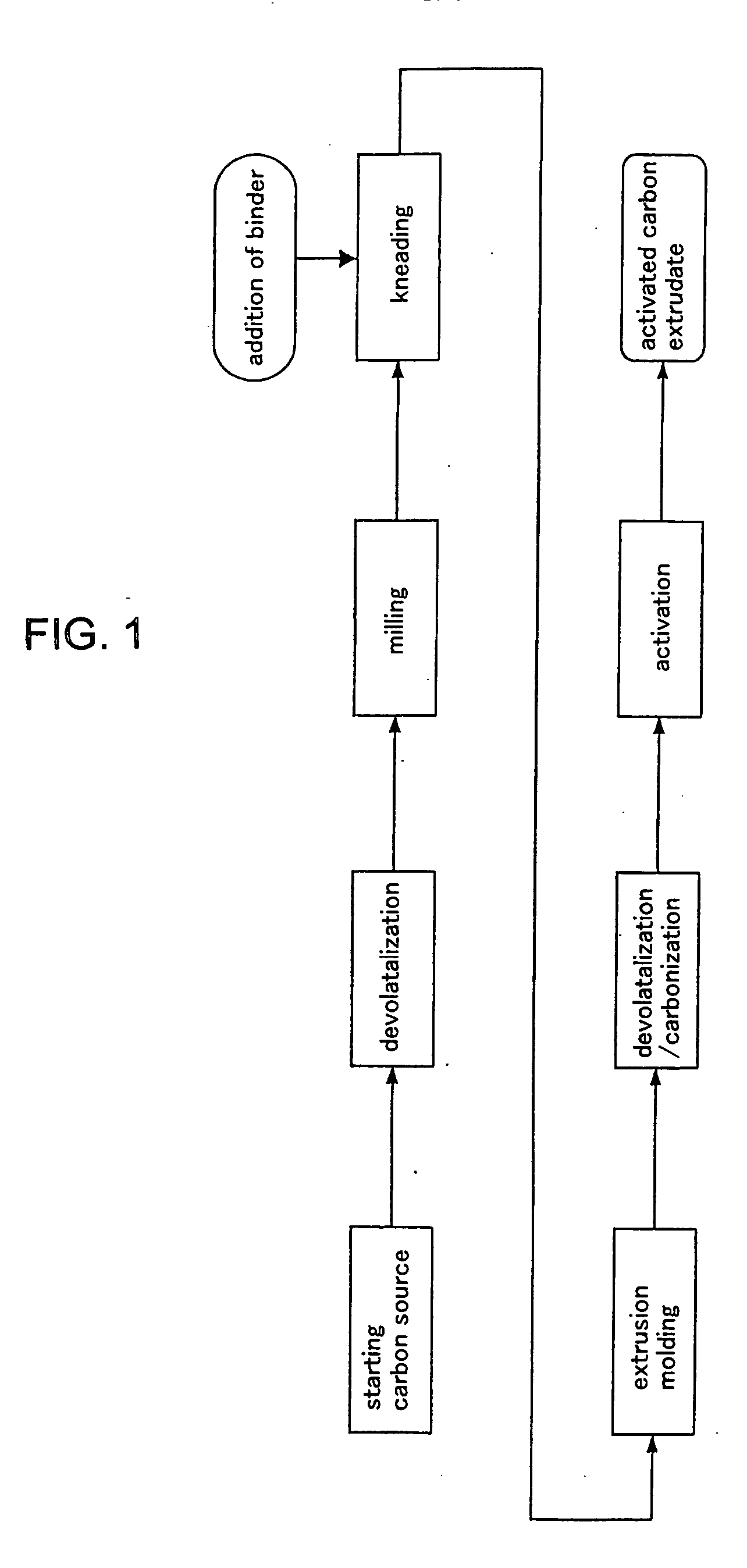
[0069] Various kinds of activated carbon were prepared by the above-described method of producing activated carbon extrudate.
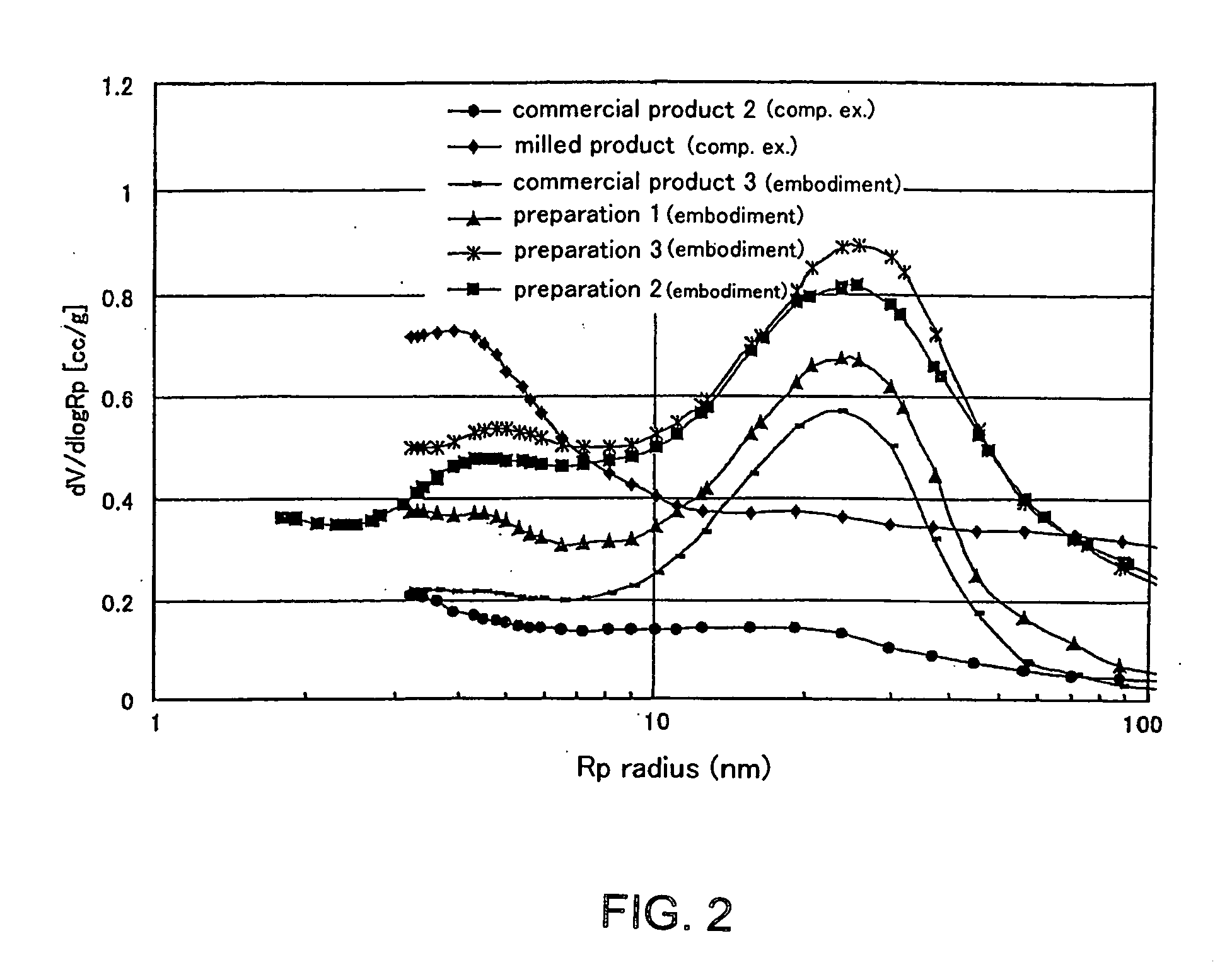
[0070] The states of Preparations 1, 2 and 3 prepared in Examples 1-1,1-2 and 1-3 from Yalloum brown coal as the starting carbon source by changing the activation time are shown in Table 1. Commercial Product 1 in Comparative Example 1-1 and Commercial Product 2 in Comparative Example 1-2 are commercial activated carbon made of peat, and Commercial Product 3 in Example 1-4 is produced by activating Comparative Product 2 (activated carbon) again with steam for 3 hours to change its pore structure. Australian Yalloum brown coal could be used as the carbon source to produce activated carbon wherein the ratio by volume of pores having pore diameter in the range of 38 to 90 nm to pores having pore diameter in the range of 20 to 200 nm was 45% or more.
[0071] A patent application (JP-A2001-9282) concerning activated carbon as a catalyst for hydrocracking of heavy o...
example 2
[0075] The ability of activated carbon to adsorb asphaltene was examined by the method described above for the ability to adsorb asphaltene.
[0076] Table 2 shows physical properties of activated carbon extrudate not carrying metal.
[0077] The vacuum residual oil was from the Middle East crude, and had the following main properties: API 5.35; 22.4 wt % CCR (carbon residue), 4.02 wt % sulfur, 0.53 wt % nitrogen, 53 wppm nickel, 180 wppm vanadium and 9.08 wt % asphaltene (nC7 Insols.).
[0078] As can be seen from Table 2, it was found that the activated carbon extrudate having pores with an absolute value of pore volume in the range of 0.8 ml / g or more wherein the proportion of pores having pore diameter in the range of 20 to 200 nm is 30 vol % or more, and the ratio by volume of pores having pore diameter in the range of 38 to 90 nm to pores having pore diameter in the range of 20 to 200 nm is 45% or more is also excellent in the ability to adsorb asphaltene.
TABLE 2ComparativeCompara...
example 3
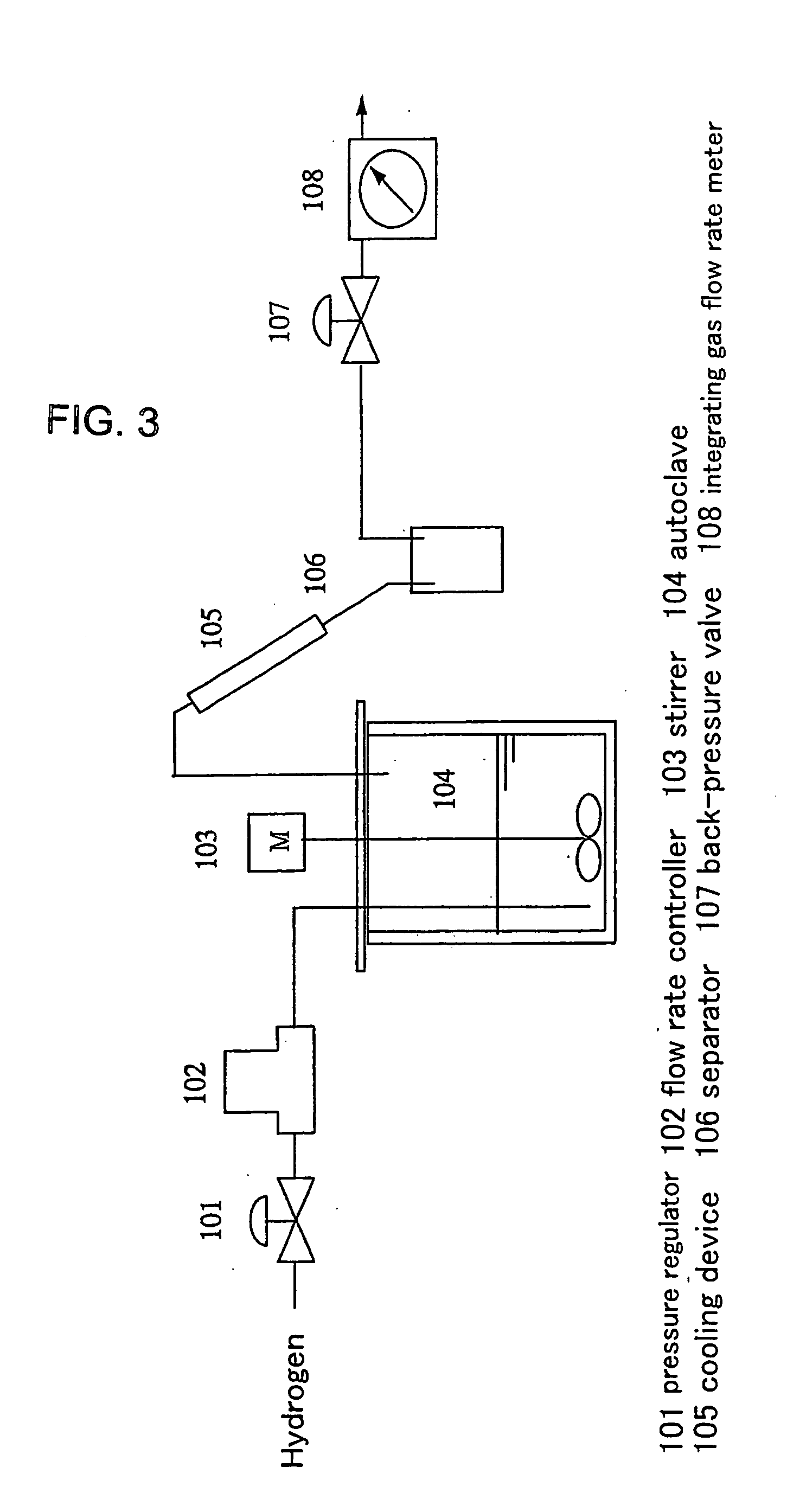
[0079] Activated carbon catalysts were prepared by the method described above for conversion of activated carbon extrudate into a catalyst, and the abilities of the activated carbon catalysts to suppress formation of coke were compared and evaluated by the method described above for the ability to suppress formation of coke.
[0080] The vacuum residual oil was from the Middle East crude, and had the following main properties: API 5.35; 22.4 wt % CCR (carbon residue), 4.02 wt % sulfur, 0.53 wt % nitrogen, 53 wppm nickel, 180 wppm vanadium and 9.08 wt % asphaltene (nC7 Insols.).
[0081] As shown in Table 3, it was found that the activated carbon extrudate having pores with an absolute value of pore volume in the range of 0.8 ml / g or more wherein the proportion of pores having pore diameter in the range of 20 to 200 nm is 30 vol % or more, the ratio by volume of pores having pore diameter in the range of 38 to 90 nm to pores having pore diameter in the range of 20 to 200 nm is 45% or mor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com