Substrates and compounds bonded thereto
a technology applied in the field of substrates and compounds bonded thereto, can solve the problems of incomplete or inefficient immobilization of nucleophile-containing materials on the substra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0149] A functionalized porous membrane coated with diamond-like glass (DLG) was prepared. A 5 cm2 high density polyethylene thermally induced phase separation (HDPE TIPS) membrane (obtained from 3M Company, St. Paul, Minn.) with a pore size of about 0.09 um and having a thickness of about 23 micrometers was diamond like glass (DLG) coated, using a plasma process as described in EP 1 266 045 B1 (David et al) to extend the DLG coating into the pores of the TIPS membrane. The DLG-coated TIPS membrane was placed in 50 ml of ethanol containing 2% by volume 3-amino propyl triethoxy silane (Sigma-Aldrich, St. Louis, Mo.), 1 ml water and few drops of 0.1N acetic acid. After 10 minutes in this solution the membrane was removed and washed with ethanol and dried.
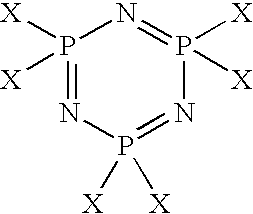
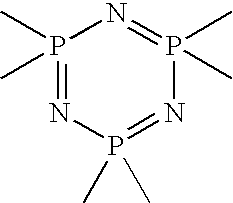
[0150] A PNC trimer was tethered on the functionalized membrane by placing the membrane in 20 ml of a toluene solution containing 0.2 g of PNC (Sigma Aldrich, St. Louis, Mo.) which was purified by sublimation. The amino group of the ...
example 2
[0154] Glass slides were treated with DLG using the following conditions. Each glass slide was etched in oxygen plasma for 10 seconds and exposed to a mixture of tetramethylsilane and oxygen plasma for 20 seconds followed by oxygen plasma for another 10 seconds. The DLG coated glass slides were then placed in a 1% solution of 3-aminopropyltriethoxy silane in ethanol for 10 minutes. Thereafter, the glass slides were removed and washed with ethanol and dried under a nitrogen flow. The dried glass slides were reacted with phosphoric chloride in toluene (Sigma Aldrich, St. Louis, Mo.). The reaction time was varied from several minutes up to one hour. Contact angle measurements were taken to monitor and confirm attachment of the PNC to the aminopropyltriethoxy silane attached to the DLG substrate. The amine has a low contact angle of 20 degrees, which on reaction with PNC increases to 45 degrees and which stabilized in about 10 minutes. Contact angle data for the attachment of the PNC is...
example 3
[0156] An approximately 20 cm by 30 cm polyimide film (obtained from E.I. du Pont de Nemours & Co., Wilmington, Del. under the trade designation “KAPTON E”) was first coated with diamond-like carbon (DLC) followed by diamond-like glass (DLG). The polyimide film was affixed to the powered electrode of a Model 2480 parallel-plate capacitively coupled reactive ion etcher (Plasma Therm, St. Petersburg, Fla.) using 3M 811 adhesive tape (3M Company, St. Paul, Minn.). DLC was deposited onto the polyimide membrane using an acetylene plasma. The ion etcher chamber was closed and the chamber was pumped to a pressure of 0.67 Pa (0.005 Torr). Oxygen gas was introduced into the chamber at a flow rate of 500 standard cm3 per minute, and the pressure of the chamber was maintained at 6.7 Pa (0.050 Torr). Plasma was ignited and was sustained at a power of 2000 W for 15 seconds. The oxygen gas flow was then terminated and the chamber was allowed to pump to a pressure of 0.67 Pa (0.005 Torr). Acetylen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pore size | aaaaa | aaaaa |
| Pore size | aaaaa | aaaaa |
| Pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com