Electroless cobalt alloy deposition process
a cobalt alloy and deposition process technology, applied in the direction of liquid/solution decomposition chemical coating, metallic material coating process, coating, etc., can solve the problems of not satisfactorily catalyzing or initiating, reducing the reliability of the overall circuit of the formed device, and not being able to follow cu-cmp processes. , to achieve the effect of reducing copper corrosion and improving the initiation delay of capping layer deposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
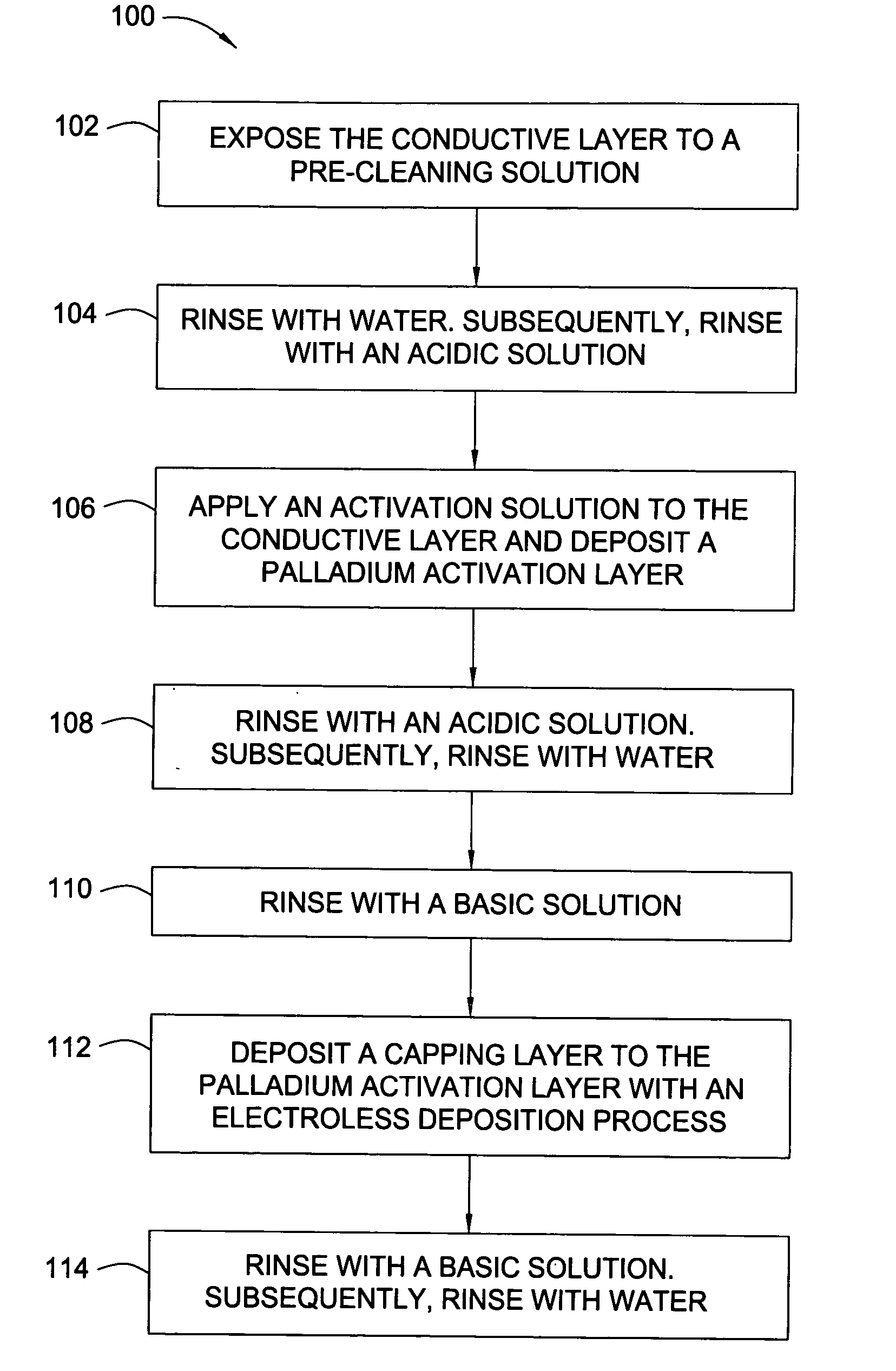
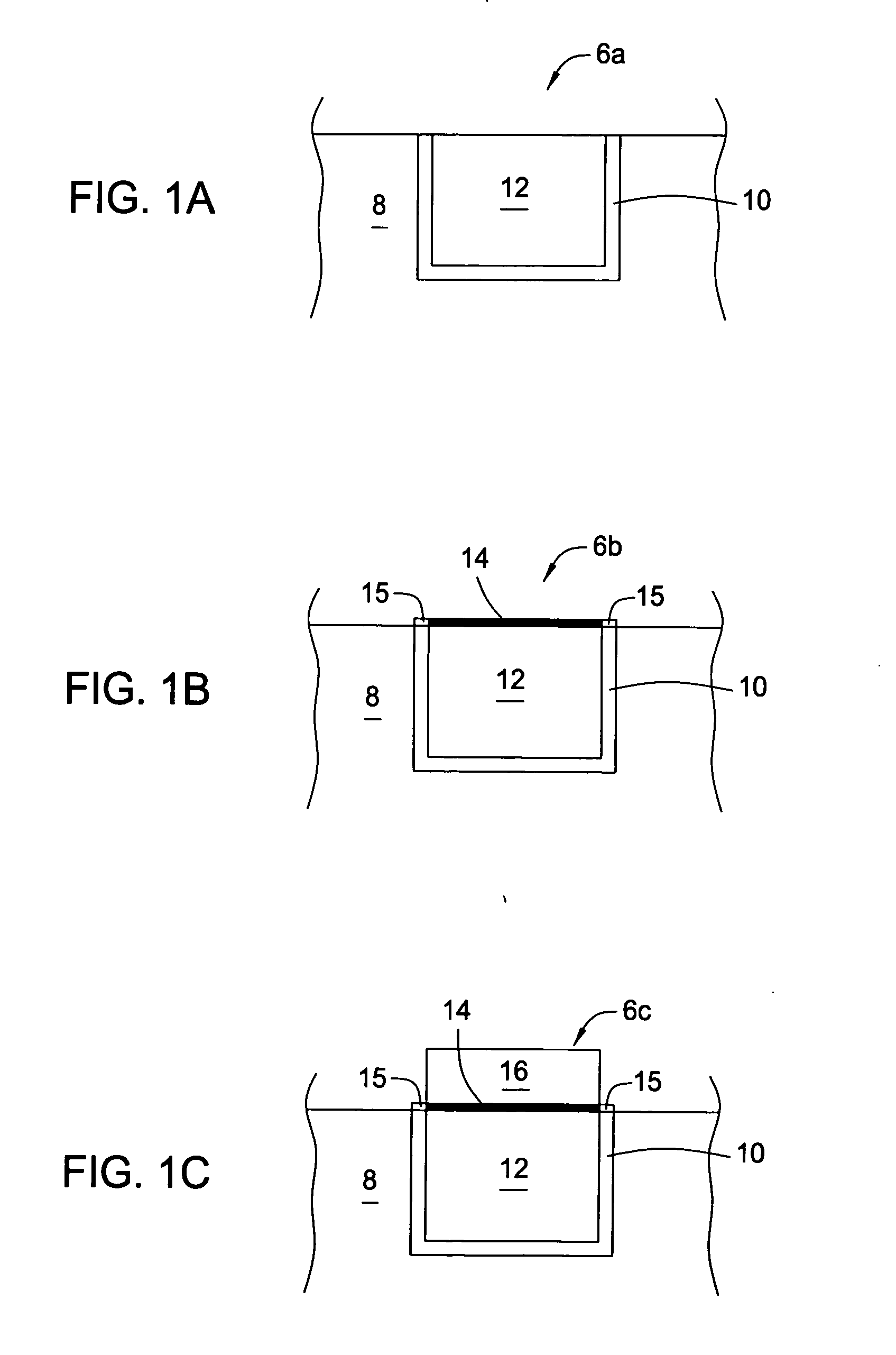
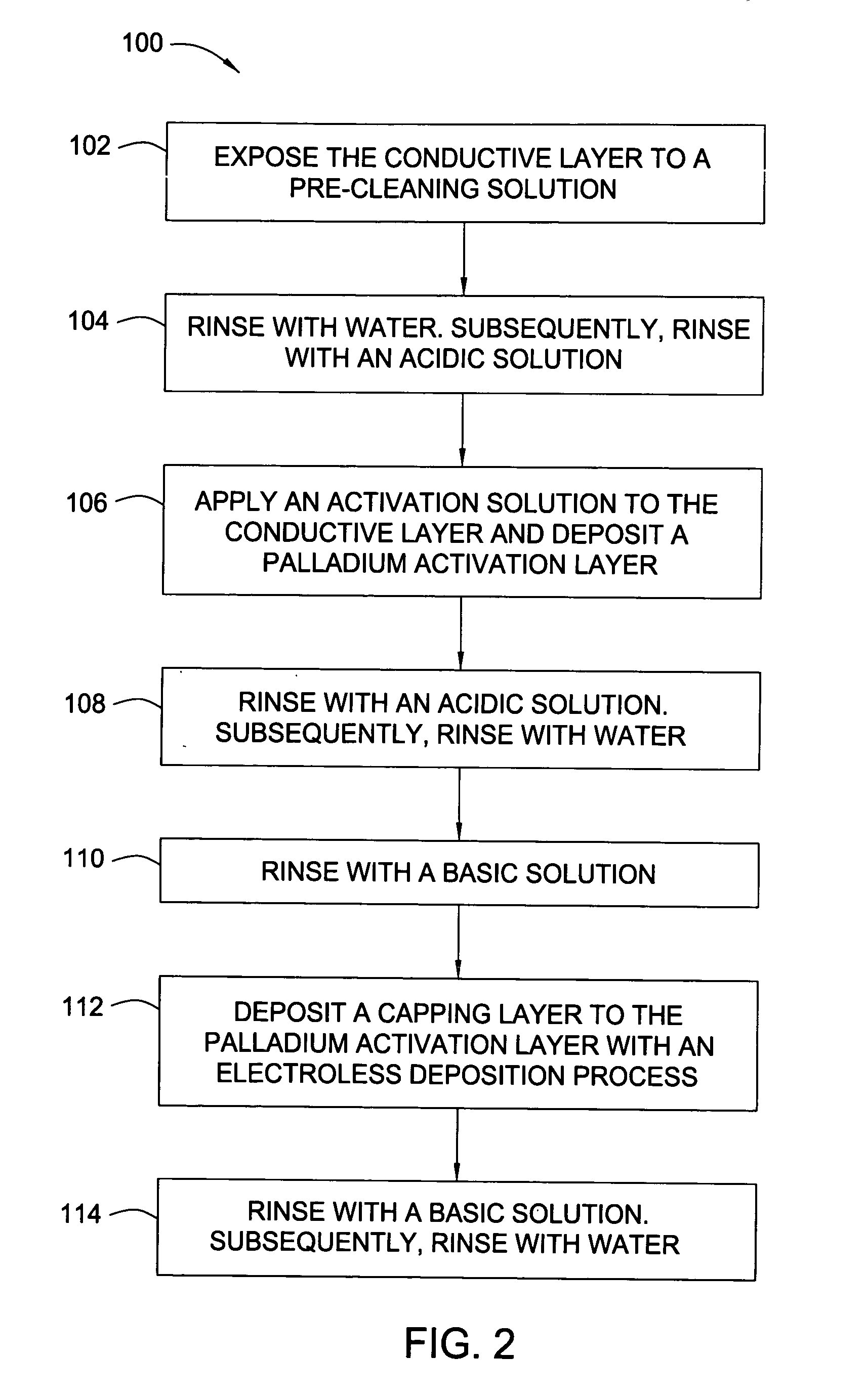
[0064] After a CMP process, a 300 mm substrate containing copper filled features supported by TaN / Ta barrier layers was rinsed with degassed, deionized water, exposed to a complexing solution for 30 seconds and subsequently rinsed with degassed, deionized water for 30 seconds. The substrate was exposed to an acidic wash containing HNO3 with a pH of 2.8. The acidified substrate was exposed for 60 seconds to 200 mL of a palladium activation solution (pH of 2.8) containing 0.04 mM Pd(NO3)2 and 1.0 mM HNO3. The substrate was rinsed with the acid wash and subsequently rinsed with degassed, deionized water for 30 seconds. The rinsed substrate was exposed to a pH basic wash solution containing TMAH for 20 seconds. The basified palladium layer was exposed to an electroless cobalt-containing solution containing 25 mg / L of surfactant (TRITON® 100) and 100 mg / L of ascorbic acid to form a capping layer. The substrate was rinsed with the pH basic wash solution and subsequent degassed, deionized ...
example 2
[0065] After a CMP process, a 300 mm substrate containing copper filled features supported by TaN / Ta barrier layers was rinsed with degassed, deionized water, exposed to a complexing solution for 30 seconds and subsequently rinsed with degassed, deionized water for 30 seconds. The substrate was exposed to an acidic wash containing HNO3 with a pH of 2.5. The acidified substrate was exposed for 40 seconds to 200 mL of a palladium activation solution (pH of 2.5) containing 0.87 mM Pd(NO3)2 and 2.0 mM HNO3. The substrate was rinsed with the acid wash and subsequently rinsed with degassed, deionized water for 30 seconds. The rinsed substrate was exposed to a pH basic wash solution containing TMAH for 20 seconds. The basified palladium layer was exposed to an electroless cobalt-containing solution containing 25 mg / L of surfactant (TRITON® 100) and 100 mg / L of ascorbic acid to form a capping layer. The substrate was rinsed with the pH basic wash solution and subsequent degassed, deionized ...
example 3
[0066] After a CMP process, a 300 mm substrate containing copper filled features supported by TaN / Ta barrier layers was rinsed with degassed, deionized water, exposed to a complexing solution for 30 seconds and subsequently rinsed with degassed, deionized water for 30 seconds. The substrate was exposed to an acidic wash containing HNO3 with a pH of 2.9. The acidified substrate was exposed for 60 seconds to 200 mL of a palladium activation solution (pH of 2.9) containing 0.04 mM Pd(NO3)2 and 1.0 mM methanesulfonic acid. The substrate was rinsed with the acid wash and subsequently rinsed with degassed, deionized water for 30 seconds. The rinsed substrate was exposed to a pH basic wash solution containing TMAH for 20 seconds. The basified palladium layer was exposed to an electroless cobalt-containing solution containing 25 mg / L of surfactant (TRITON® 100) and 100 mg / L of ascorbic acid to form a capping layer. The substrate was rinsed with the pH basic wash solution and subsequent dega...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| exposure time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com