Prokaryotically produced antibodies and use thereof
a technology of prokaryotically produced antibodies and antibodies, which is applied in the field of molecular biology and protein technology, can solve the problems of poor yield of reconstituted tetrameric antibodies, i>e. coli /i>would not be a useful system for making intact antibodies, and both approaches have limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of Expression Vectors
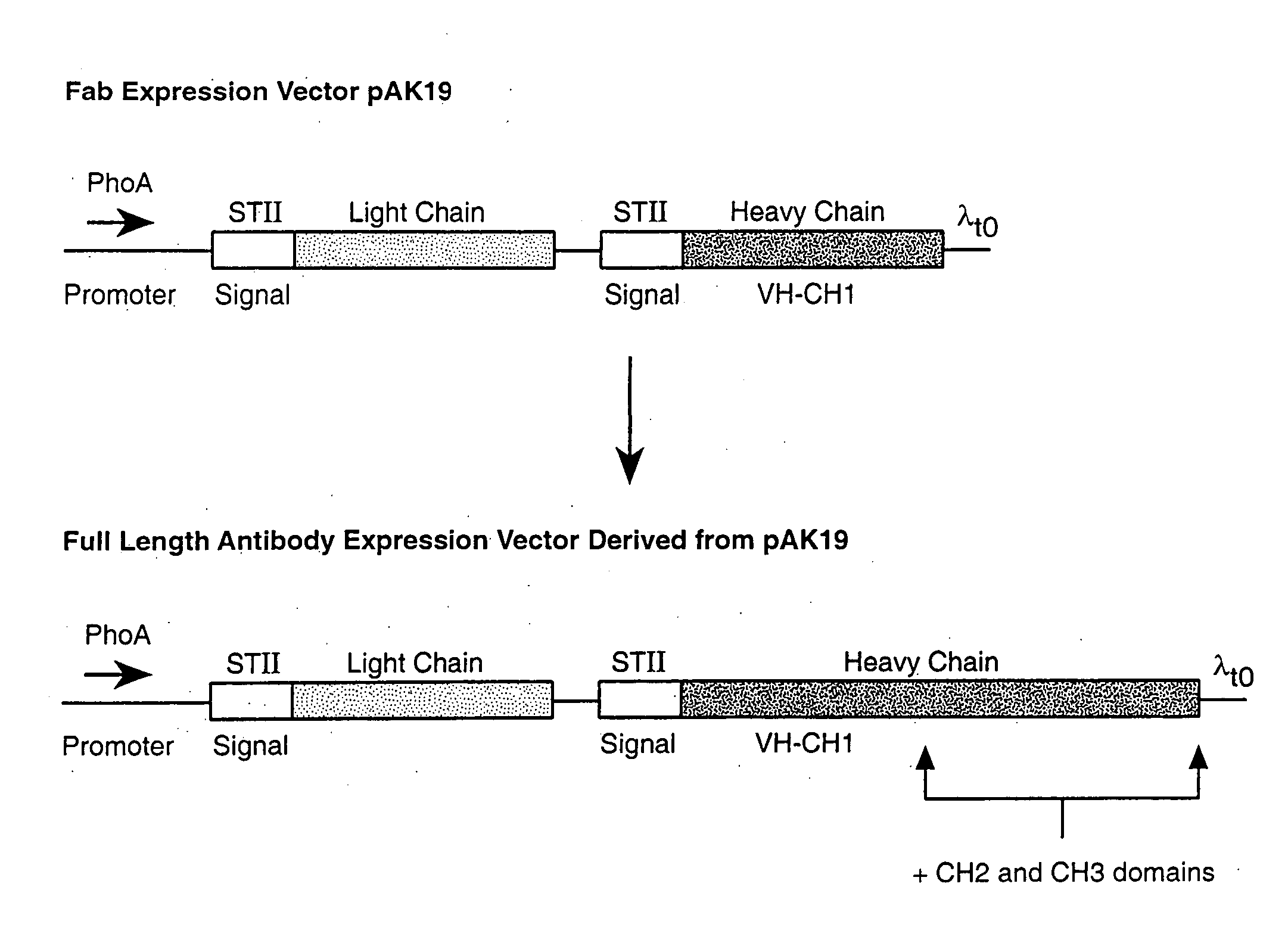
[0165] Various expression vectors were made for the expression of antibodies specific to tissue factor (anti-TF antibody) and antibodies specific to vascular endothelial cell growth factor (anti-VEGF antibody). For each vector construction, an expression cassette was cloned into the framework of the E. coli plasmid pBR322 at the EcoRI site. Sutcliffe (1978) Cold Spring Harbor Symp. Quant. Biol. 43:77-90. Each expression cassette contains at least the following components: (1) a phoA promoter for the control of transcription; (2) a Shine-Dalgarno sequence from the E. coli trp or the heat stable enterotoxin 11 (STII) gene, or a combination of both, for translation initiation; and (3) a λt0 terminator to end transcription. The basic components of bacterial expression cassettes are known in the art and have been described in, for example, Kikuchi et al., Nucleic Acids Res. 9(21):5671-5678 (1981) (for phoA promoter); Scholtissek and Grosse, Nucleic Acid...
example 2
E. coli Expression of Full Length Antibodies Using Polycistronic Vectors
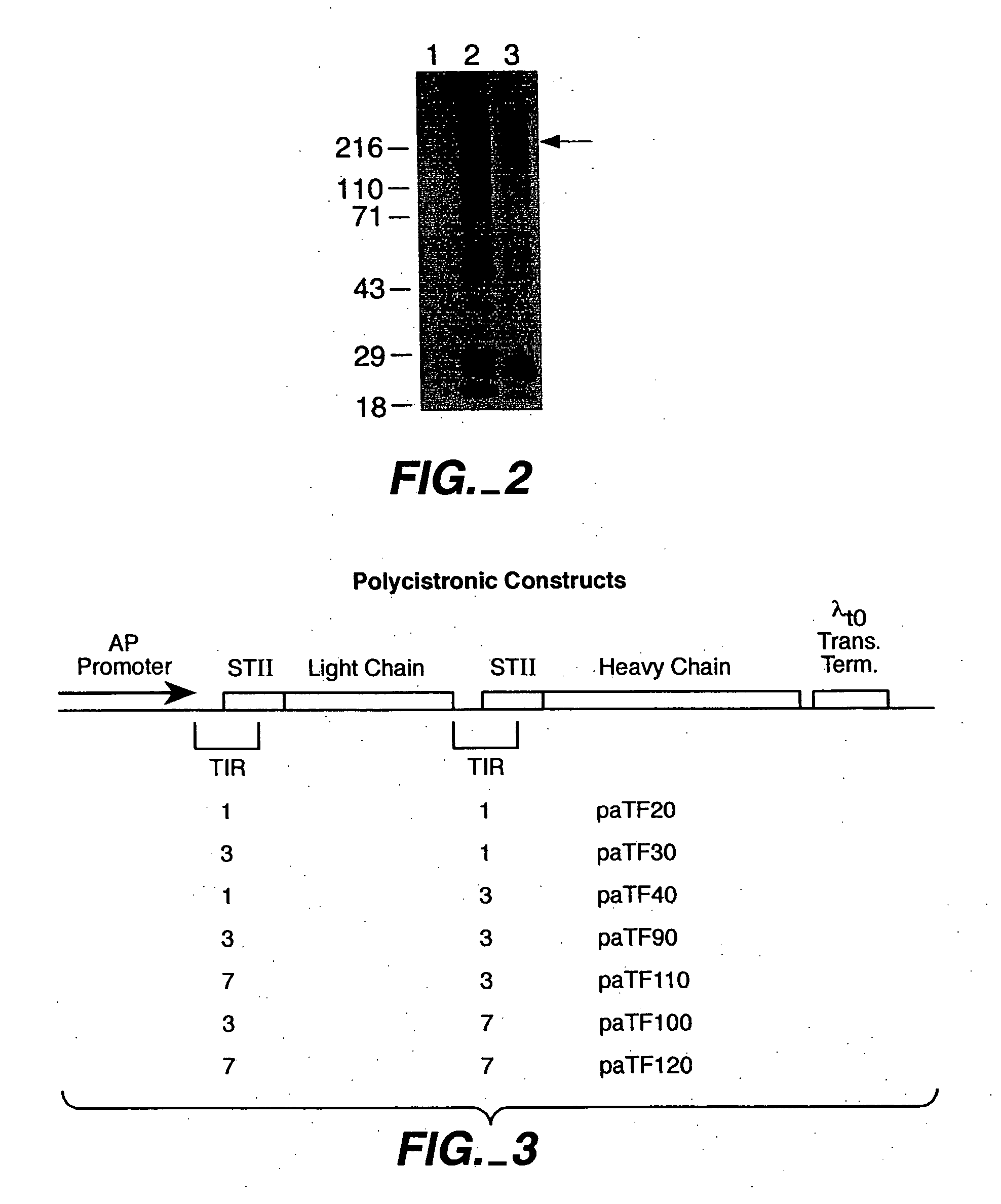
[0170] Full length antibodies were first made in E. coli using polycistronic vectors derived from a published vector, pAK19, according to the methods described in Example 1. Small scale inductions were first performed to evaluate and compare the expression levels obtained with the various constructs.
Materials and Methods
[0171] For small scale expression of each construct, the E. coli strain 33D3 (W3110 ΔfhuA (ΔtonA) ptr3 lac Iq lacL8 ΔompTΔ(nmpc-fepE) degP41 kanR) was used as host cells. Following transformation, selected transformant picks were inoculated into 5 ml Luria-Bertani medium supplemented with carbenicillin (50 ug / ml) and grown at 30° C. on a culture wheel overnight. Each culture was then diluted (1:50 or 1:100) into C.R.A.P. phosphate-limiting media (3.57 g (NH4)2SO4, 0.71 g NaCltrate-2H2O, 1.07 g KCl, 5.36 g Yeast Extract (certified), 5.36 g HycaseSF-Sheffield, adjusted pH with KOH to 7.3, qs to...
example 3
E. coli Expression of Full length Antibodies Using Separate Cistron Vectors
[0181] To construct the separate cistron vectors with modulated TIR strength combinations, a preferred TIR strength for secretion of each individual chain was first determined in a series of single cistron plasmids constructed to express light or heavy chain only (FIG. 5). A series of single cistron plasmids with various TIRs was therefore constructed for the individual expression of both anti-TF light and heavy chains. Methods and materials used for vector construction and protein expression were similar to those used for polycistronic vector expressions, which has been described in Examples 1 and 2 above.
[0182] The range of TIR strengths tested extended from a relative strength of I to a maximum relative strength of 13. Reduced whole cell lysates from induced cultures transformed with these constructed plasmids were analyzed by SDS-PAGE and the results are shown in FIG. 6. For both heavy and light chain, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com