Prevention of dissolution of metal-based aluminium production anodes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Comparative
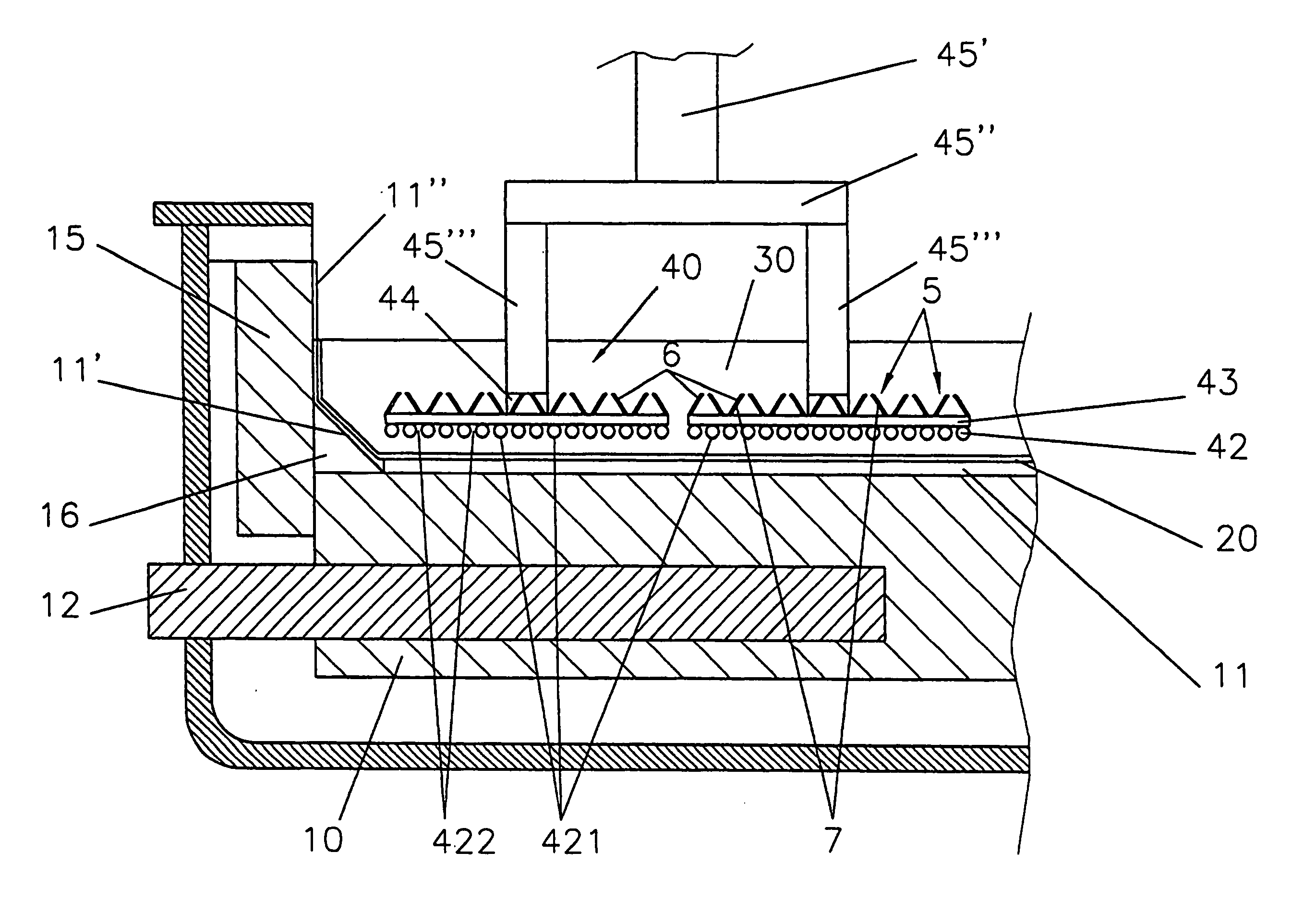
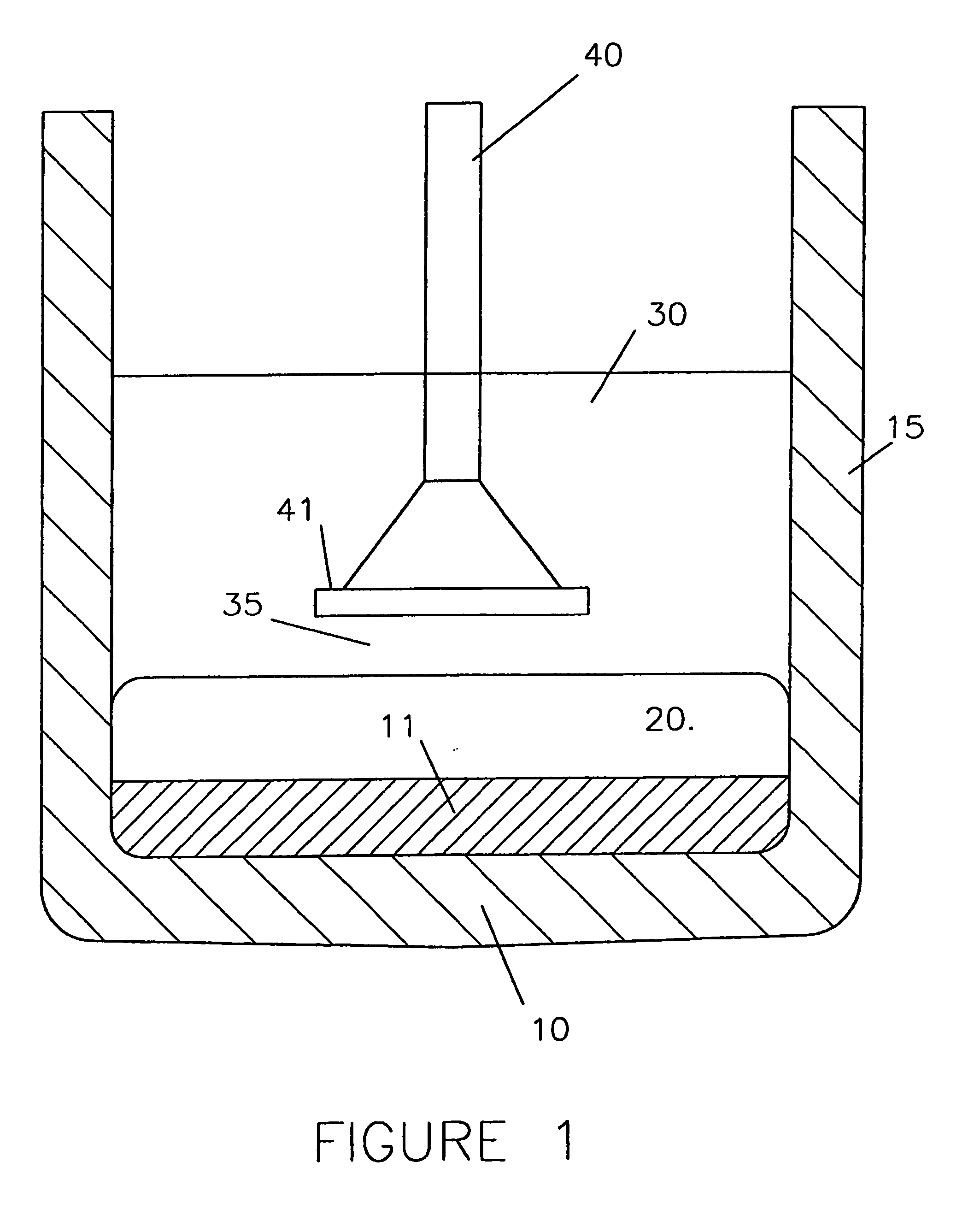
[0145] One of the above identical nickel-iron alloy anodes 40 was used in a cell, as shown in FIG. 1, having cathodically polarised carbon sidewalls 15 exposed to the molten electrolyte 30.
[0146] The electrolytic bath 30 consisted of 16 weight % AlF3, 4 weight % caF2 and 6 to 6.5 weight % dissolved Al2O3, the balance being cryolite (Na3AlF6), and was at a temperature of 930° C. The aluminium layer 20 had a thickness of about 3 cm.
[0147] Electrolysis was performed at constant current corresponding to an anodic current density of 0.8 A / cm2 whereby oxygen was anodically evolved and aluminium 20 cathodically produced by electrolysis of the dissolved alumina.
[0148] The composition of the bath 30 was analysed every 12 hours by x-ray fluorescence (XRF). The Al2O3 content in the bath was maintained substantially constant by adding every 15 min an amount of Al2O3 adjusted according to the analysed composition of the bath 30.
[0149] During the first 24 hours the cell voltage wa...
example 2
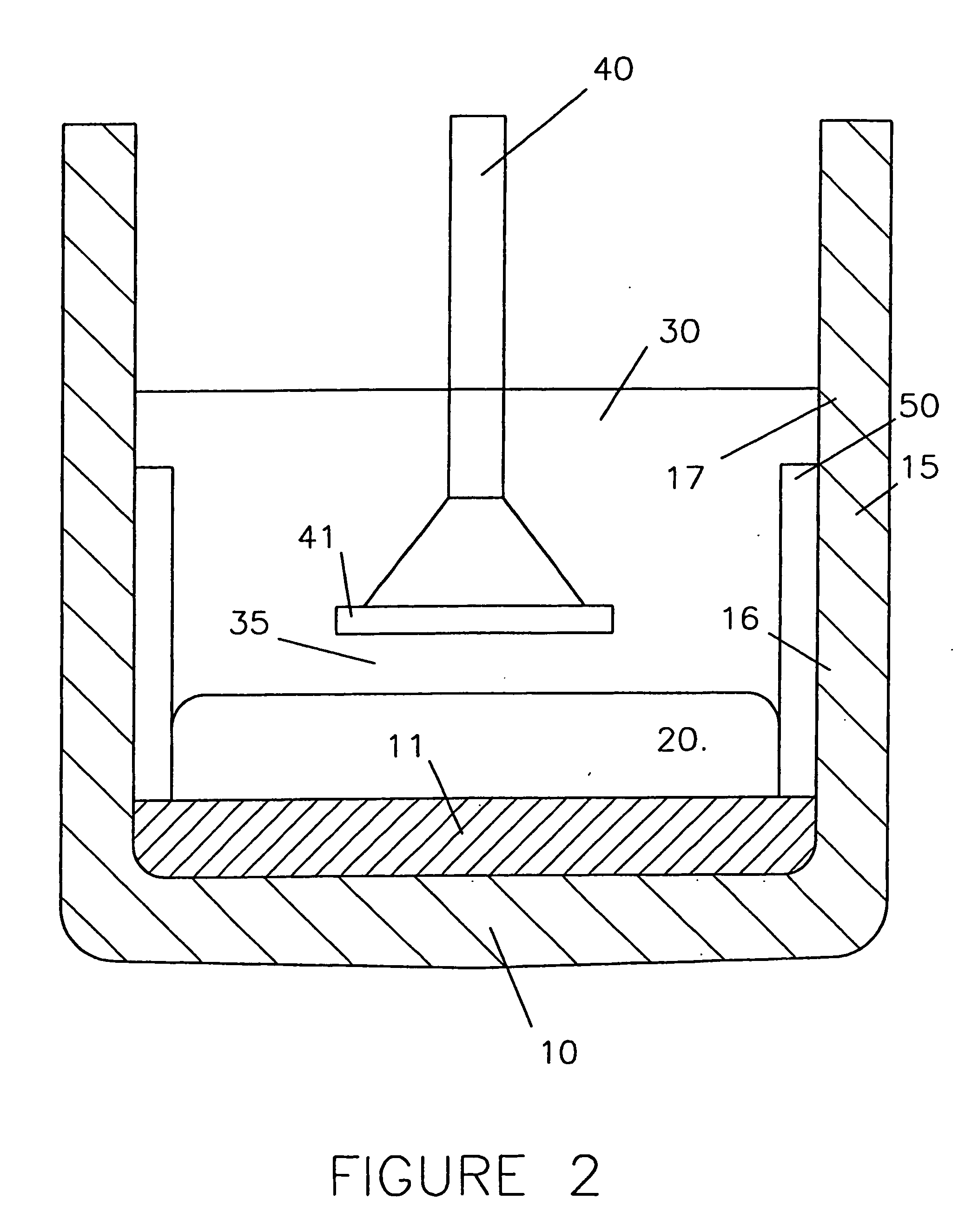
[0155] Another of the above identical nickel-iron alloy anodes was used in a cell, as shown in FIG. 2, having cathodically non-polarised upper parts 17 of carbon sidewalls 15 exposed to the molten electrolyte 30, the cathodically polarised sidewall bottom parts 16 being shielded from the electrolyte by fused alumina sleeve 50.
[0156] The electrolysis was carried out under the same operating conditions as in Example 1.
[0157] Like in the previous Example, during the first 24 hours the cell voltage was stable at 3.6 volts and the Al2O3 consumption corresponded to about 60% of the theoretical value.
[0158] After this initial period the cell voltage continued to remain substantially stable. However, the Al2O3 consumption decreased. After 50 hours the Al2O3 consumption had stabilised at 50% of the theoretical value.
[0159] After 100 hours the anode 40 was removed from the bath 30 and examined.
[0160] The external dimensions of the anode 40 had not significantly changed. The wear of the a...
example 3
[0165] The last anode of the above identical nickel-iron alloy anode was used in a cell, as shown in FIG. 3, in which no carbon is exposed to the electrolyte 30.
[0166] The electrolysis was carried out under the same operating conditions as in Examples 1 and 2.
[0167] The cell voltage was stable at 3.6 volts, and the Al2O3 consumption corresponded to about 60% of the theoretical value throughout the test.
[0168] After 100 hours the anode was removed for examination. The external dimensions of the anode were substantially unchanged.
[0169] The external dimensions of the anode 40 had not significantly changed. The wear of the anode 40 led to a reduction of the average diameter of the metallic core by 0.3 mm from 20 to 19.7 mm, which is even better than in Example 2. The anode was covered by a dense and coherent oxide scale of about 200 microns thick. No noticeable anode corrosion was observed.
[0170] The improvement of the anode wear rate between Examples 2 and 3 is believed to be due...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com