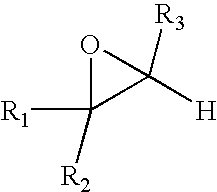
Photocurable resin composition and resin composition for plastics comprising the same
a technology of resin composition and photocurable resin, which is applied in the field of photocurable resin composition, can solve the problems of insufficient curing rate, poor adhesive property of materials, and inability to exhibit the necessary curing rate,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0045] To a 2-L acrylic resin composition preparation apparatus equipped with a stirrer, a thermometer, a reflux cooler and a dropping bath, which was under a nitrogen atmosphere, 340.0 g of xylene was put, and then the temperature was elevated to a polymerization temperature of 135° C. Herein, a mixture of 400 g of methyl methacylate, 50 g of styrene and 50 g of 2-hydroxyethyl methacrylate as radically polymerizable monomers, and 20 g of tert-butyl-2-ethyl hexanoate as a radical initiator was added dropwise over 4 hours. After completion of dropping, the reaction mixture was continuously stirred at 135° C. for 1 hour. Subsequently, xylene and remaining monomers were distilled off by having the preparation apparatus in vacuo to give an acrylic resin (B).
preparation examples 2 to 6 and 8
[0046] Reaction was performed in the same manner as in Preparation Example 1, except that the amounts of the radically polymerizable monomer and radical initiator were modified as shown in Table 1.
preparation example 7
[0047] Reaction was performed in the same manner as in Preparation Example 1, except that the amounts of the radically polymerizable monomer and radical initiator were modified and the polymerization temperature was modified to 100° C. as shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com