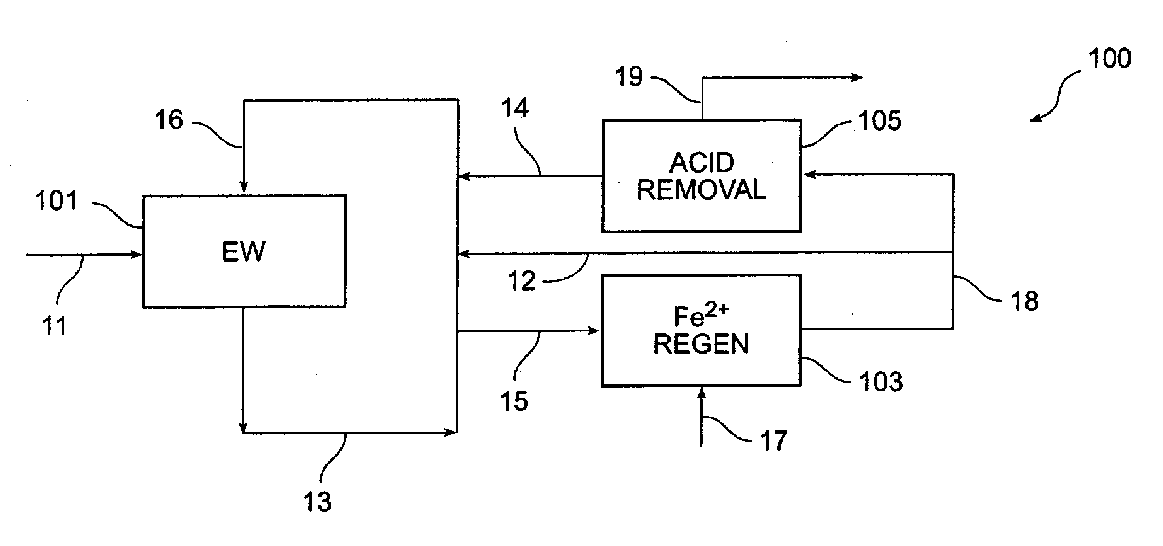
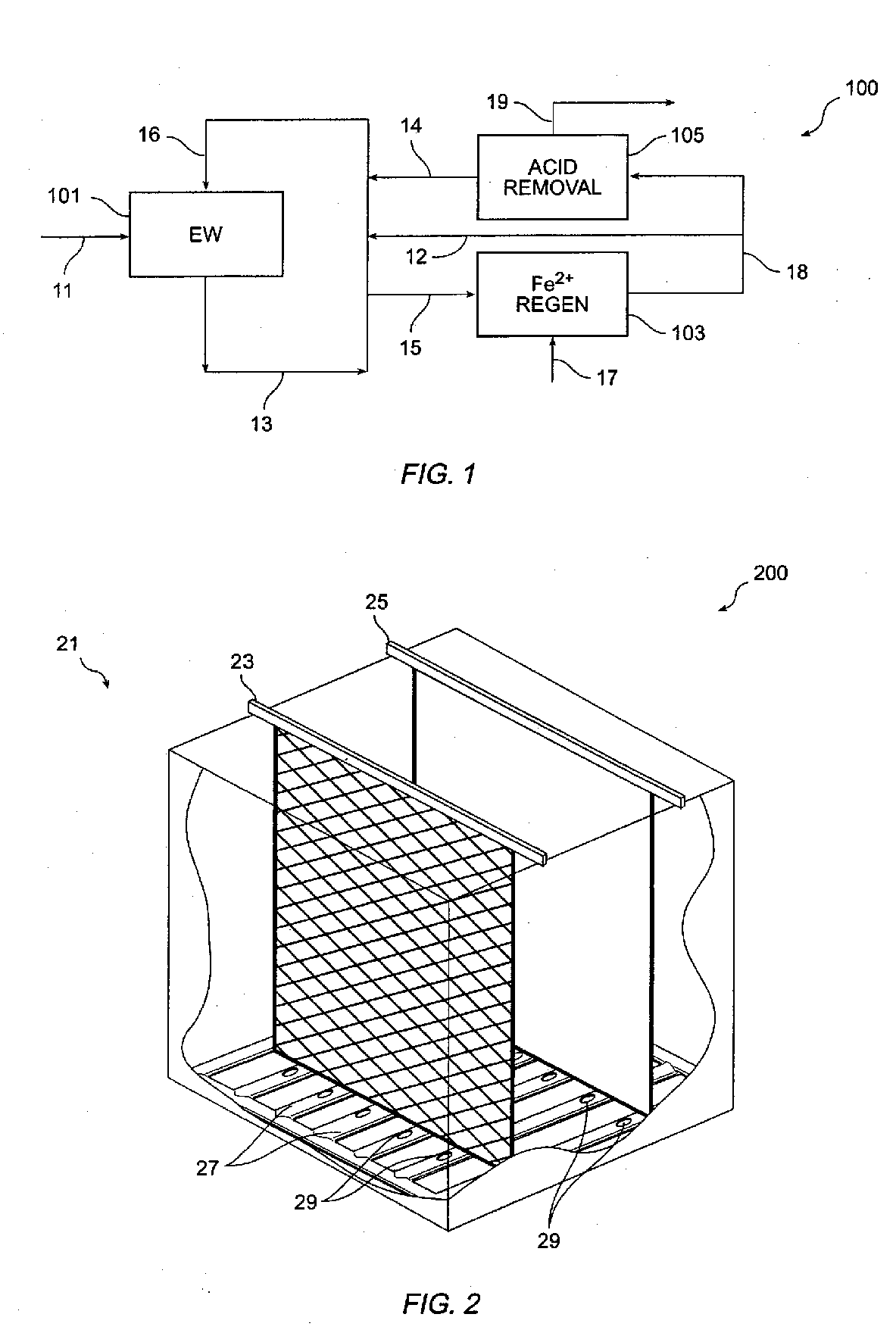
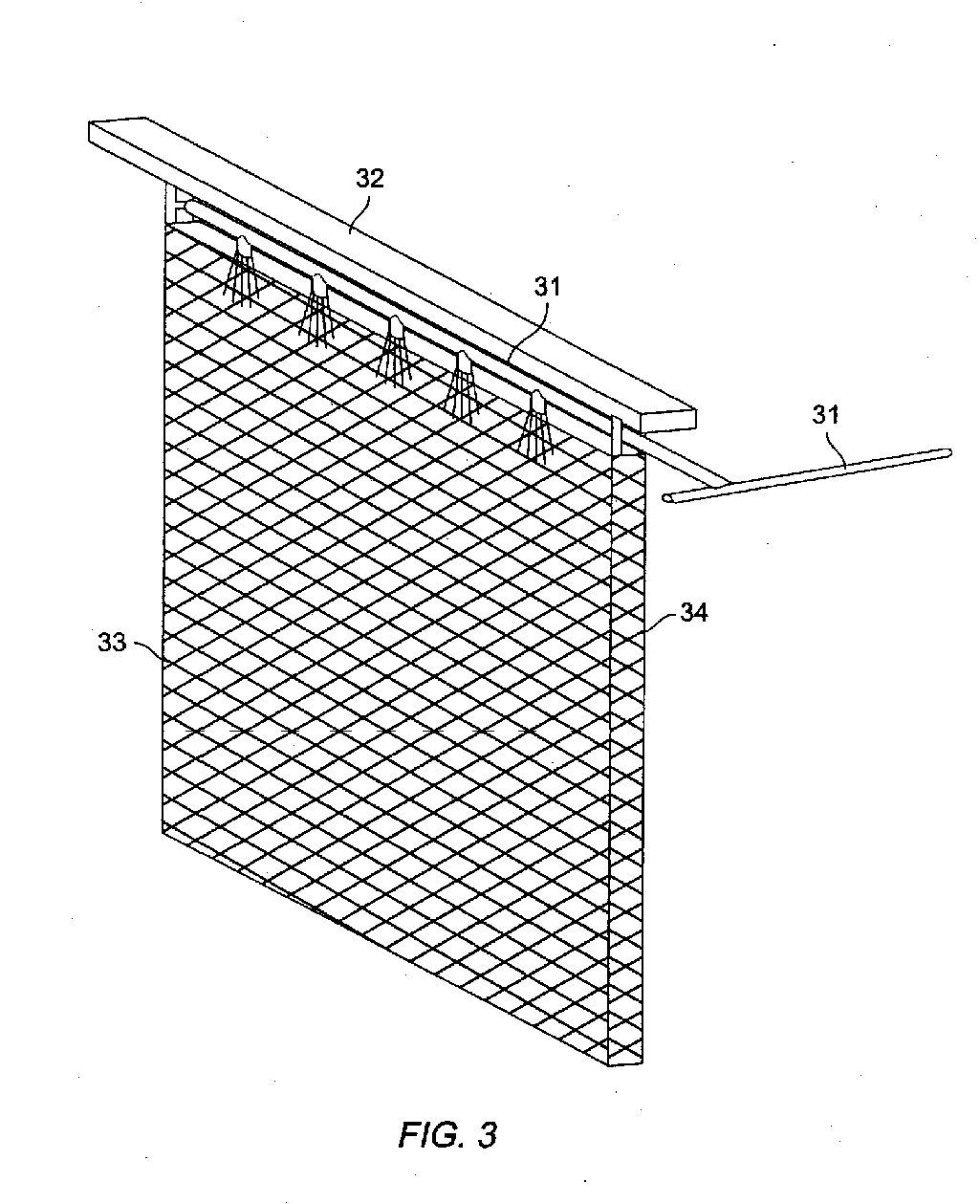
Method and apparatus for electrowinning copper using the ferrous/ferric anode reaction and a flow-through anode
a technology of ferrous/ferric anode and flow-through anode, which is applied in the field of electrowinning metals, can solve the problems of affecting the cost-effectiveness of the electrowinning process, the inability to achieve maximum voltage reduction and thus maximum energy reduction using the ferrous/ferric anode reaction, and the limitations of operating current density, so as to improve the electrowinning efficiency and reduce the generation of acid mist. , the effect of reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0095] TABLE 1 demonstrates the advantages of a flow-through anode with in-anode electrolyte injection for achieving low cell voltage. An in-anode manifold produces a lower cell voltage at the same flow or decreases flow requirements at the same current density versus bottom injection. TABLE 1 also demonstrates that a cell voltage below 1.10 V is achievable at a current density of about 35 A / ft2 (377 A / m2) and a cell voltage below 1.25 V is achievable at a current density of about 40 A / ft2 (430 A / m2).
[0096] Test runs A-F were performed using an electrowinning cell of generally standard design, comprising three full-size conventional cathodes and four full-size flow-through anodes. The cathodes were constructed of 316 stainless steel and each had an active depth of 41.5 inches and an active width of 37.5 inches (total active surface area of 21.6 ft2 per cathode). Each anode had an active width of 35.5 inches and an active depth of 39.5 inches and was constructed of titanium mesh wit...
example 2
[0105] TABLE 2 demonstrates that increasing temperature decreases cell voltage.
[0106] Test runs A-C were performed using an electrowinning cell of generally standard design, comprising three full-size conventional cathodes and four full-size flow-through anodes. The cathodes were constructed of 316 stainless steel and each had an active depth of 41.5 inches and an active width of 37.5 inches (total active surface area of 21.6 ft2 per cathode). Each anode had an active width of 35.5 inches and an active depth of 39.5 inches and was constructed of titanium mesh with an iridium oxide-based coating. The anodes used in accordance with this EXAMPLE 2 were obtained from Republic Anode Fabricators of Strongsville, Ohio, USA.
[0107] Test duration was six days, with continuous 24-hour operation of the electrowinning cell at approximately constant conditions. Voltage measurements were taken once per day using a handheld voltage meter and voltages were measured bus-to-bus. The stated values fo...
example 3
[0114] TABLE 3 demonstrates the effectiveness of a graphite foam and two titanium-graphite sintered anodes. The first titanium-graphite sintered anode comprised of 12% graphite and 88% titanium. The second titanium-graphite sintered anode comprised of 8% graphite and 92% titanium. The titanium-graphite sintered anodes were prepared by mixing powders of the graphite and titanium and pressing them into a hollow cylinder. The hollow cylinders were then attached to a titanium rod or a co-extruded copper-titanium rod. The rods with attached cylinders are then sintered to provide mechanical strength. Finally, the rods are hung from a copper hanger bar to form the anode.
[0115] The present inventors have demonstrated that use of such anodes in connection with an electrowinning process using the ferrous / ferric anode reaction enables an average cell voltage of less than 1.0 V. Approximately 3-inch by 5-inch samples of such anodes were immersed in copper-containing electrolyte solution and at...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current density | aaaaa | aaaaa |
| voltage | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com