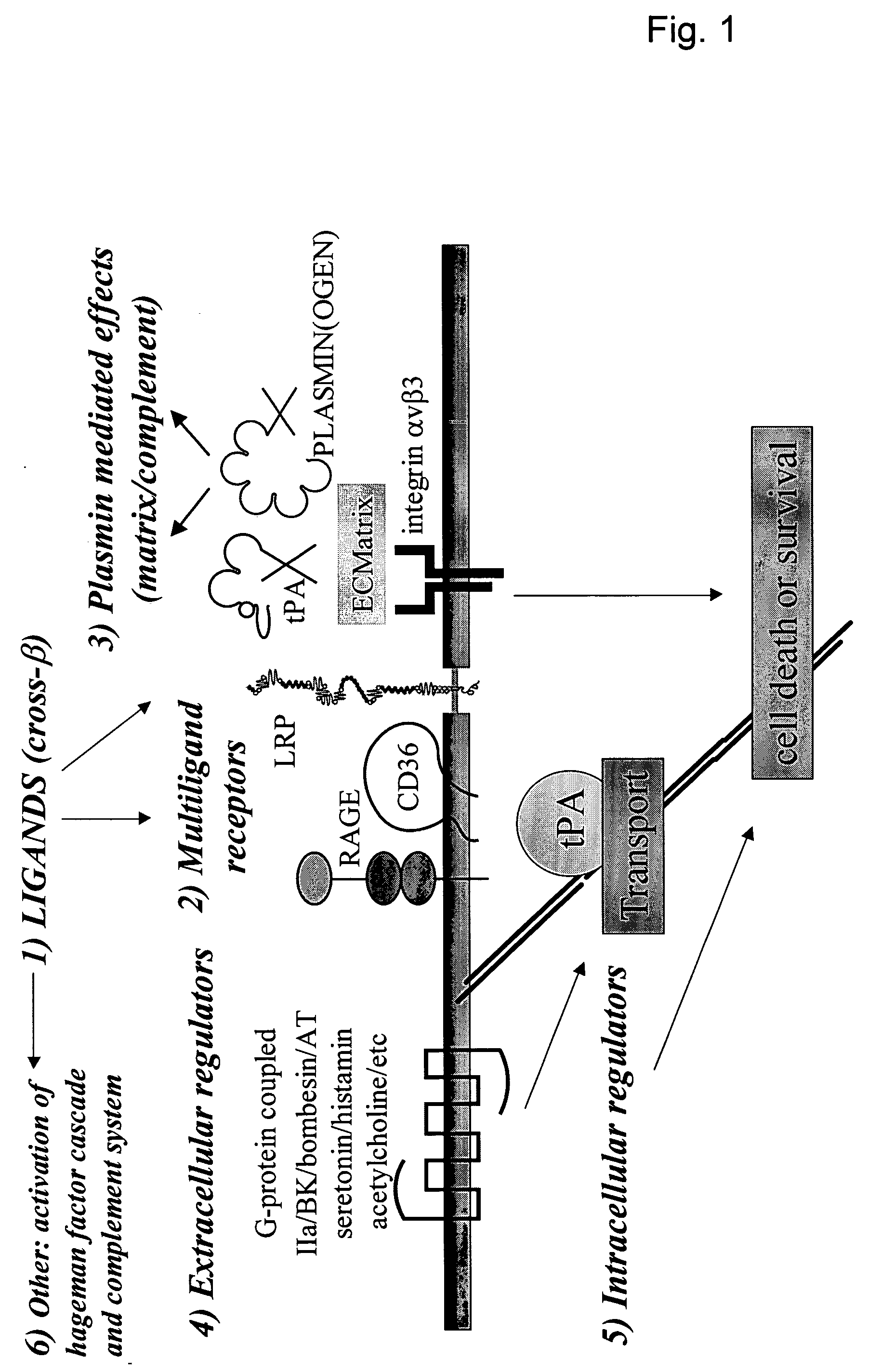
Cross-beta structure comprising amyloid-binding proteins and methods for detection of the cross-beta structure, for modulating cross-beta structures fibril formation and for modulating cross-beta structure-mediated toxicity
a cross-beta and protein technology, applied in the field of biotechnology, biochemistry, molecular biology, structural biology and medicine, can solve the problems of unknowing the why and how of all of these proteins, and achieve the effect of increasing local cytotoxicity and/or fibrinolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Reagents
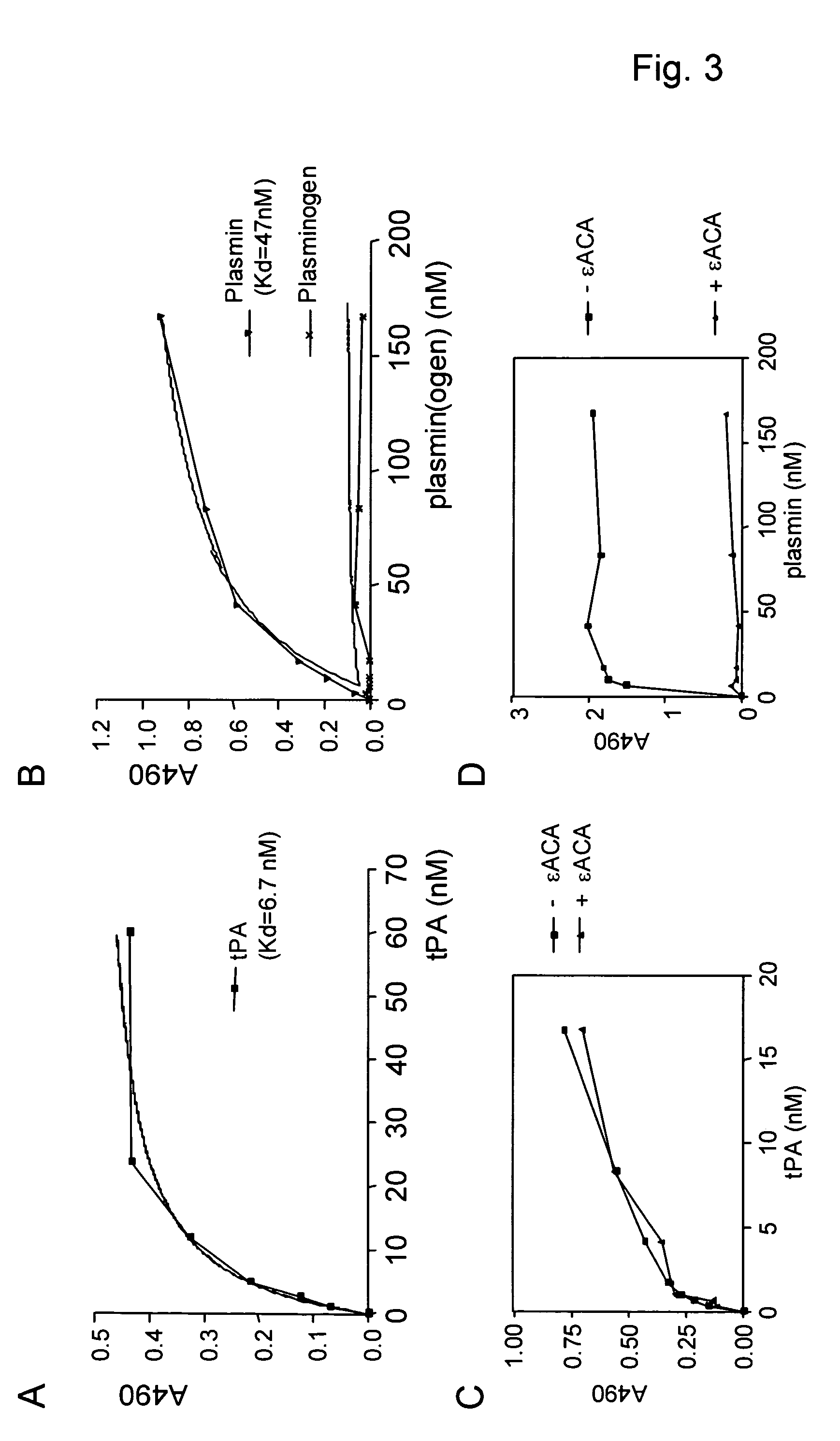
[0105] Bovine serum albumin (BSA) fraction V pH 7.0 and D-glucose-6-phosphate di-sodium (g6p), D, L-glyceraldehyde, and chicken egg-white lysozyme were from ICN (Aurora, Ohio, USA). Rabbit anti-recombinant tissue-type plasminogen activator (tPA) 385R and mouse anti-recombinant tPA 374B were purchased from American Diagnostica (Veenendaal, The Netherlands). Anti-laminin (L9393) was from Sigma. Swine anti-rabbit immunoglobulins / HRP (SWARPO) and rabbit anti-mouse immunoglobulins / HRP (RAMPO) were from DAKO Diagnostics B.V. (The Netherlands). Alteplase (recombinant tissue-type plasminogen activator, tPA) was obtained from Boehringer-Ingelheim (Germany). Reteplase (Rapilysin), a recombinant mutant tPA containing only kringle 2 and the catalytic domain (K2P-tPA) was obtained from Roche, Hertfordshire, UK, and porcine pancreas carboxypeptidase B (CpB) was from Roche, Mannheim, Germany. Carboxypeptidase inhibitor (CPI) was from Calbiochem (La Jolla, Calif., USA). Tween 20 was purcha...
example 2
Synthetic Peptides
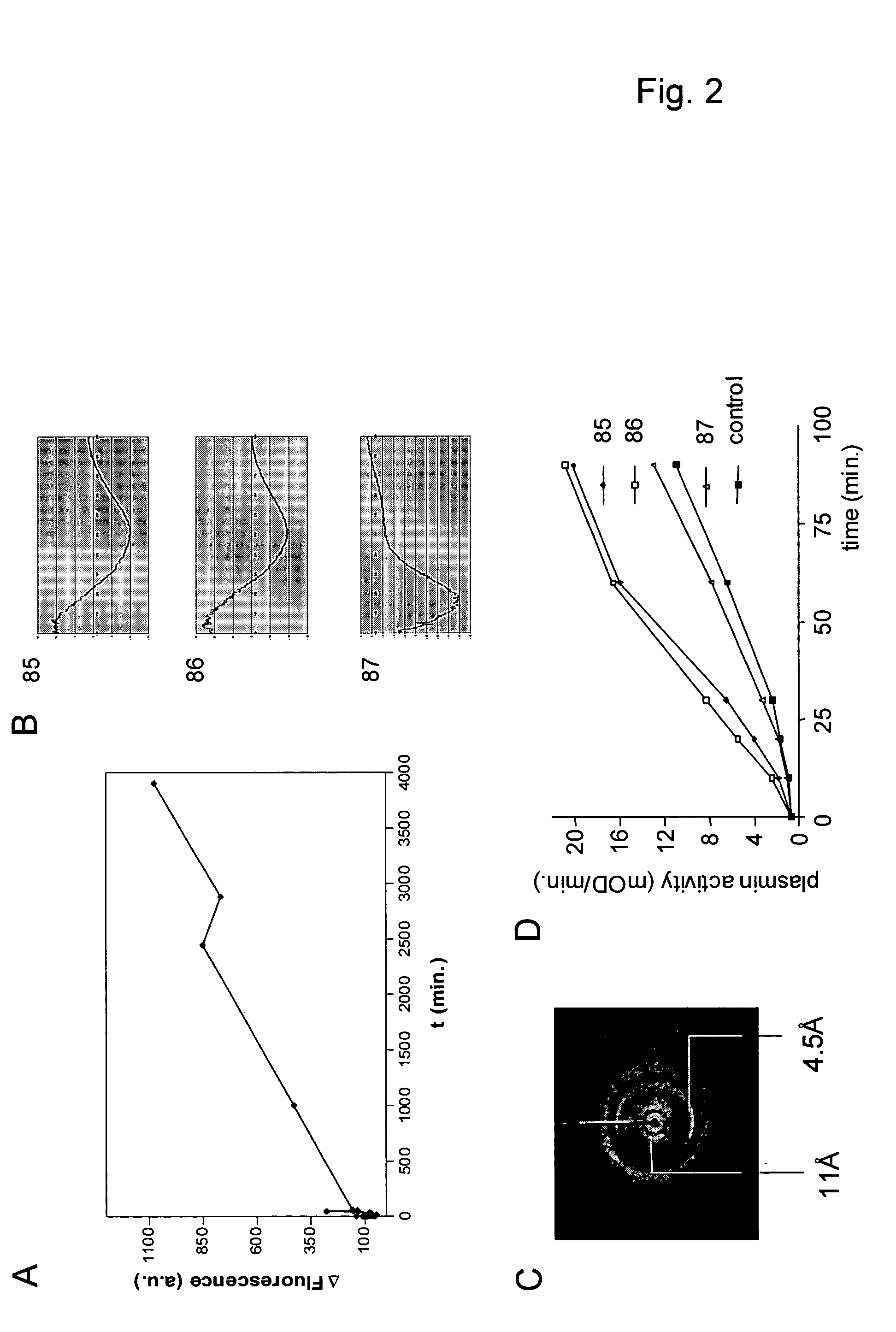
[0106] Peptide Aβ (1-40), containing amino acids as present in the described human Alzheimer peptide (DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV) (SEQ ID NO: 18), fibrin peptides 85 (or FP13) (KRLEVDIDIKIRS) (SEQ ID NO: 19), 86 (or FP12) (KRLEVDIDIKIR) (SEQ ID NO: 20) and 87 (or FP10) (KRLEVDIDIK) (SEQ ID NO: 21), derived from the sequence of human fibrin(ogen) and the islet amyloid polypeptide (IAPP) peptide or derivatives (fl-hIAPP: KCNTATCATQRLANFLVHSSNNFGAILSSTNVGSNTY (SEQ ID NO: 22), ΔhIAPP (SNNFGAILSS) (SEQ ID NO:23 ), ΔmIAPP (SNNLGPVLPP) (SEQ ID NO: 24) were obtained from Pepscan, Inc. (The Netherlands) or from the peptide synthesis facility at the Netherlands Cancer Institute (NCI, Amsterdam, The Netherlands). The peptides were dissolved in phosphate buffered saline (PBS) to a final concentration of 1 mg ml−1 and stored for three weeks at room temperature (RT) to allow formation of fibrils. During this period, the suspension was vortexed twice weekly. After ...
example 3
Congo Red Binding and Thioflavin T Fluorescence of a Fibrin Clot
[0107] For Thioflavin T-fluorescence measurements, 1 mg ml−1 of fibrinogen was incubated at 37° C. with 2 U ml−1 of factor IIa in 150 mM NaCl, 20 mM Tris-HCl pH 7.5, 10 mM CaCl2, 50 μM Thioflavin T. Background fluorescence of a clot was recorded in the absence of Thioflavin T and background Thioflavin T fluorescence was measured in the absence of factor IIa. Fluorescence was measured on a Hitachi F-4500 fluorescence spectrophotometer (Ltd., Tokyo, Japan), using Sarstedt REF67.754 cuvettes. Apparatus settings: excitation at 435 nm (slit 10 nm), emission at 485 nm (slit 10 nm), PMT voltage 950 V, measuring time 10 seconds, delay 0 seconds. For detection of Congo red binding, a fibrin clot was formed at room temperature as described above (Thioflavin T was omitted in the buffer). The clot was incubated with Congo red solution and washed according to the manufacturer's recommendations (Sigma Diagnostics, MO, USA). The clot...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| emission wavelength | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com