Use of adipose tissue-derived stromal stem cells in treating fistula
a technology of stromal stem cells and adipose tissue, which is applied in the direction of genetically modified cells, skeletal/connective tissue cells, prosthesis, etc., can solve the problems of incontinence, recurrence, reinfection, and incontinence, and achieves greater homogeneity and distinct phenotypes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Stem Cells from Lipoaspirates with Improved Homozeneity
[0101] Adipose tissue was obtained by liposuction, under local anaesthesia and general sedation. A hollow blunt-tipped cannula was introduced into the subcutaneous space through a small incision (less than 0.5 cm in diameter). With gentle suction, the cannula was moved through the adipose tissue abdominal-wall compartment for mechanical disruption of the fatty tissue. A saline solution and the vasoconstrictor epinephrine were injected into the adipose tissue compartment to minimize blood loss. In this way, 80 to 100 ml of raw of lipoaspirate were obtained from each patient to be treated.
[0102] The raw lipoaspirate was washed extensively with sterile phosphate-buffered saline (PBS; Gibco BRL, Paisley, Scotland, UK) to remove blood cells, saline and local anaesthetic. The extracellular matrix was digested with a solution of type II collagenase (0.075%; Gibco BRL) in balanced salt solution (5 mg / ml; Sigma, St. Loui...
example 2
Characterization of Stem Cells from Lipoaspirates with Improved Homogeneity
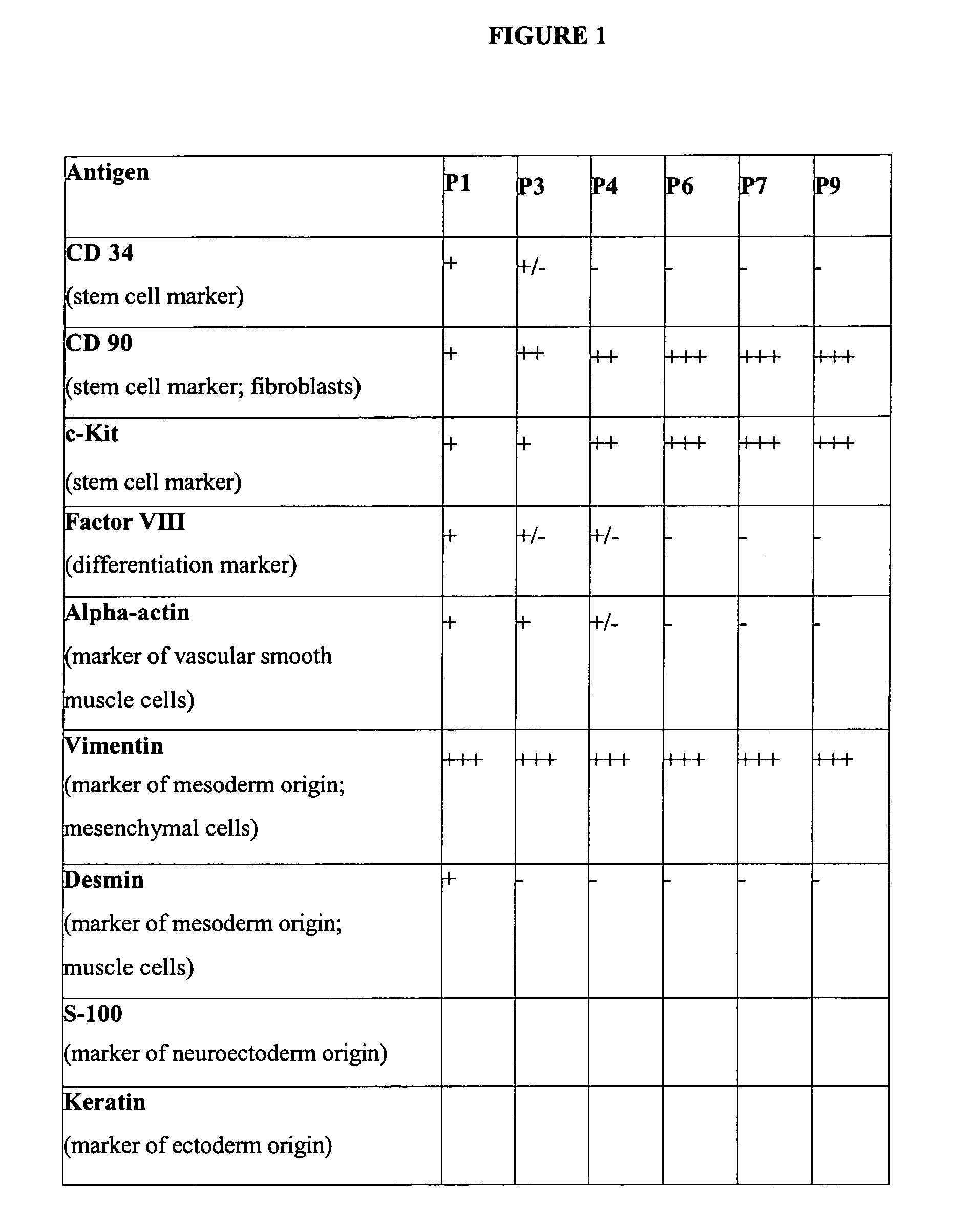
[0104] To characterize the cells by immunofluorescence staining, cells were plated at low density in DMEM plus 10% FBS on glass cover slips in 24-well plates. For immunohistochemistry studies, cells were washed with PBS and fixed in acetone for 10 min at −20° C. For staining of a-actin, cells were fixed in 4% paraformaldehyde for 10 min at RT. After blocking with a PBS that contained 4% goat serum and 0.1% Triton X-100, cells were incubated at 4° C. overnight with primary antibodies against the following cell markers at the indicated dilutions [(i) alpha-actin; Dako, Glostrup, Denmark; 1 / 50; (ii) vimentin; Sigma, St. Louis, USA; 1 / 200; (iii) CD 90; CYMBUS, Biotechnology LTD, Chandlers Ford, Hants, UK; 1 / 50; (iv) Factor VIII; Dako; 1 / 100; (v) CD 34; Chemicon, Calif., USA; 1 / 100; (vi) c-Kit; Chemicon; 1 / 100; (vii) desmin; Dako; 1 / 100; (viii) cytokeratin; Dako; 1 / 100 and (ix) S-100; Dako; 1 / 50]. Then cells were...
example 3
Preparations of Stem Cells Comprising Fibrin Glue for Use in Treating Fistula
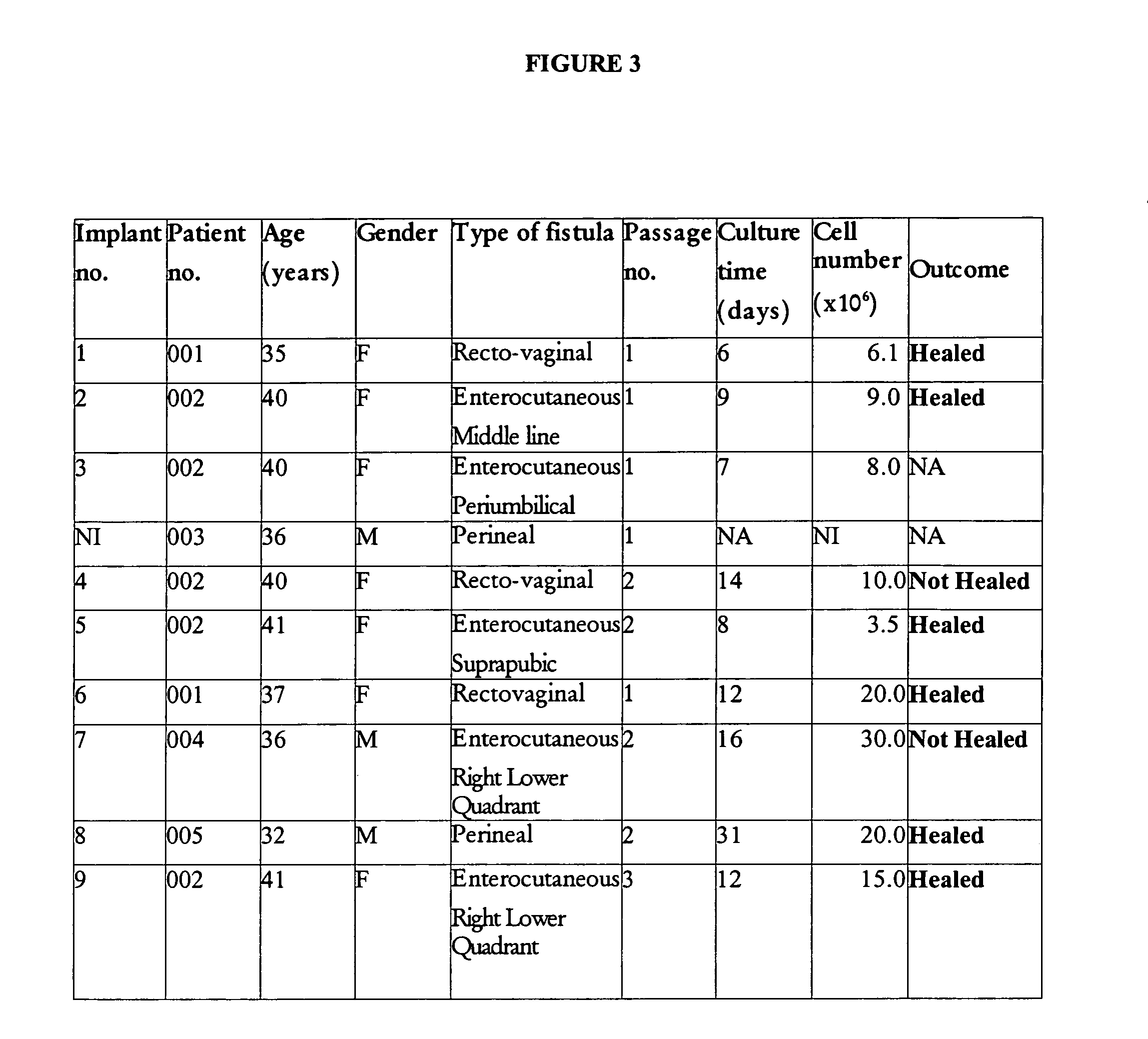
[0122] For clinical use, the cells as prepared above may be used after three or fewer passages (FIG. 3), but are preferably used after two or more passages as described above to afford a cell preparation with higher homogeneity. Cell cultures for clinical use were trypsinized for 3 min at 37° C. Trypsinization was stopped by addition of DMEM plus FBS, and the suspension was centrifuged at 110×g for 5 min. Cells were washed in PBS and the suspension was centrifuged again at 150×g for 5 min. Cell were resuspended at between 3 and 30×106 cells / ml in 1 to 2 ml of Ringer's lactate solution and put in a suitable syringe. Human serum albumin (HSA) may optionally be added to the Ringer's lactate solution.
[0123] In certain cases, half of the cells were resuspended in the thrombin component of a fibrin glue kit (Tissucol® Duo 2.0; Baxter, Madrid, Spain) prior to combination of the kit's two components, in an attemp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com