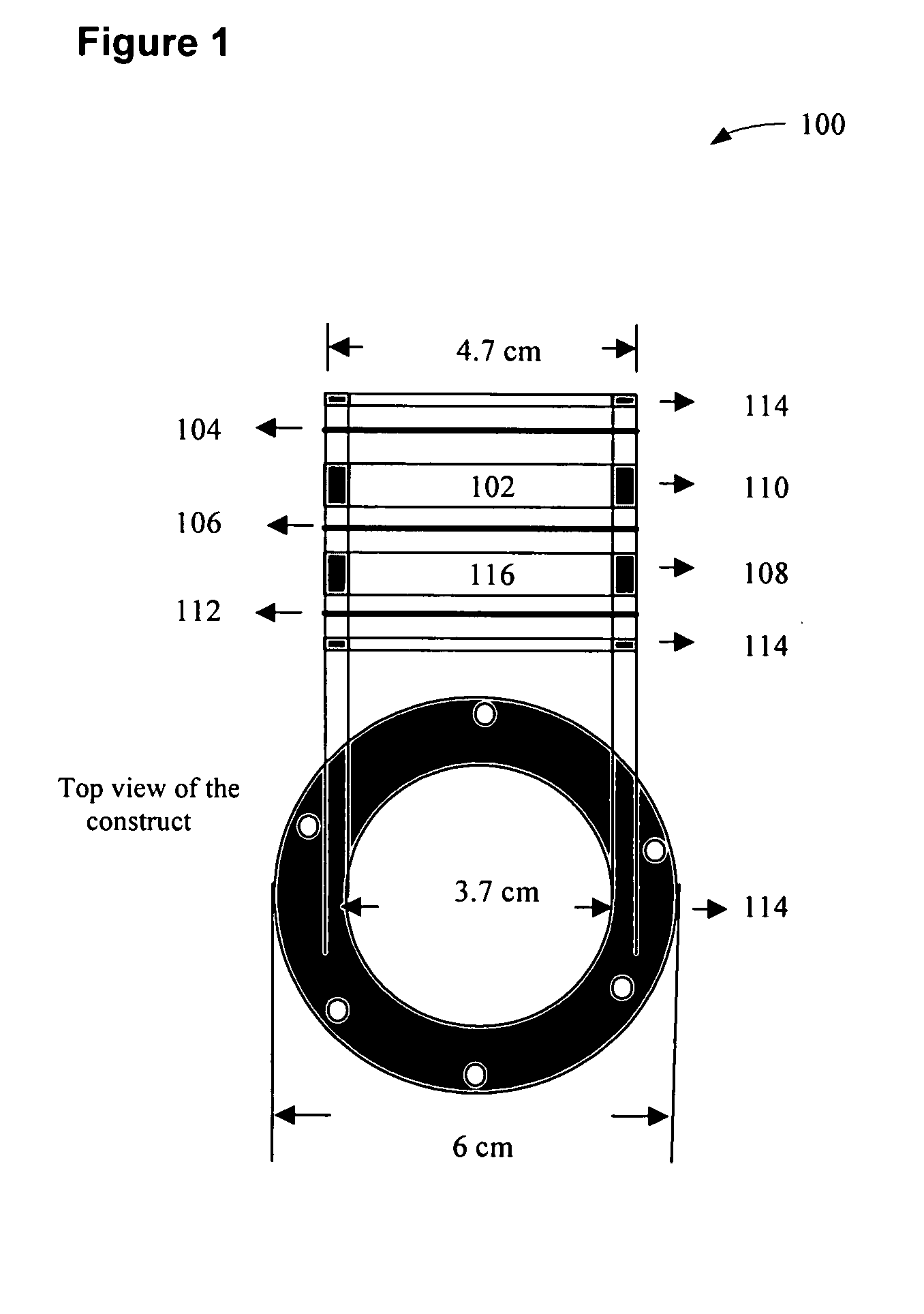
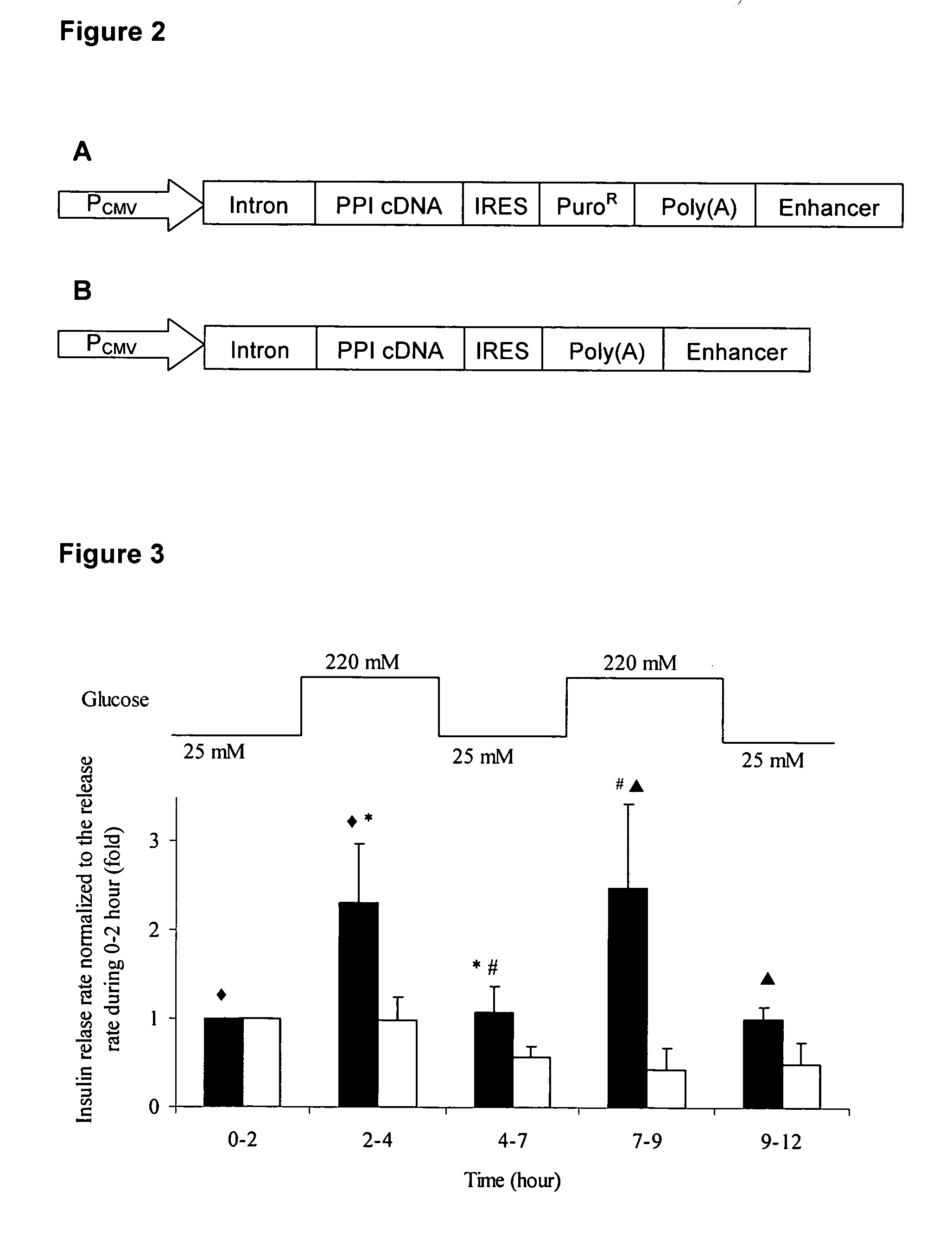
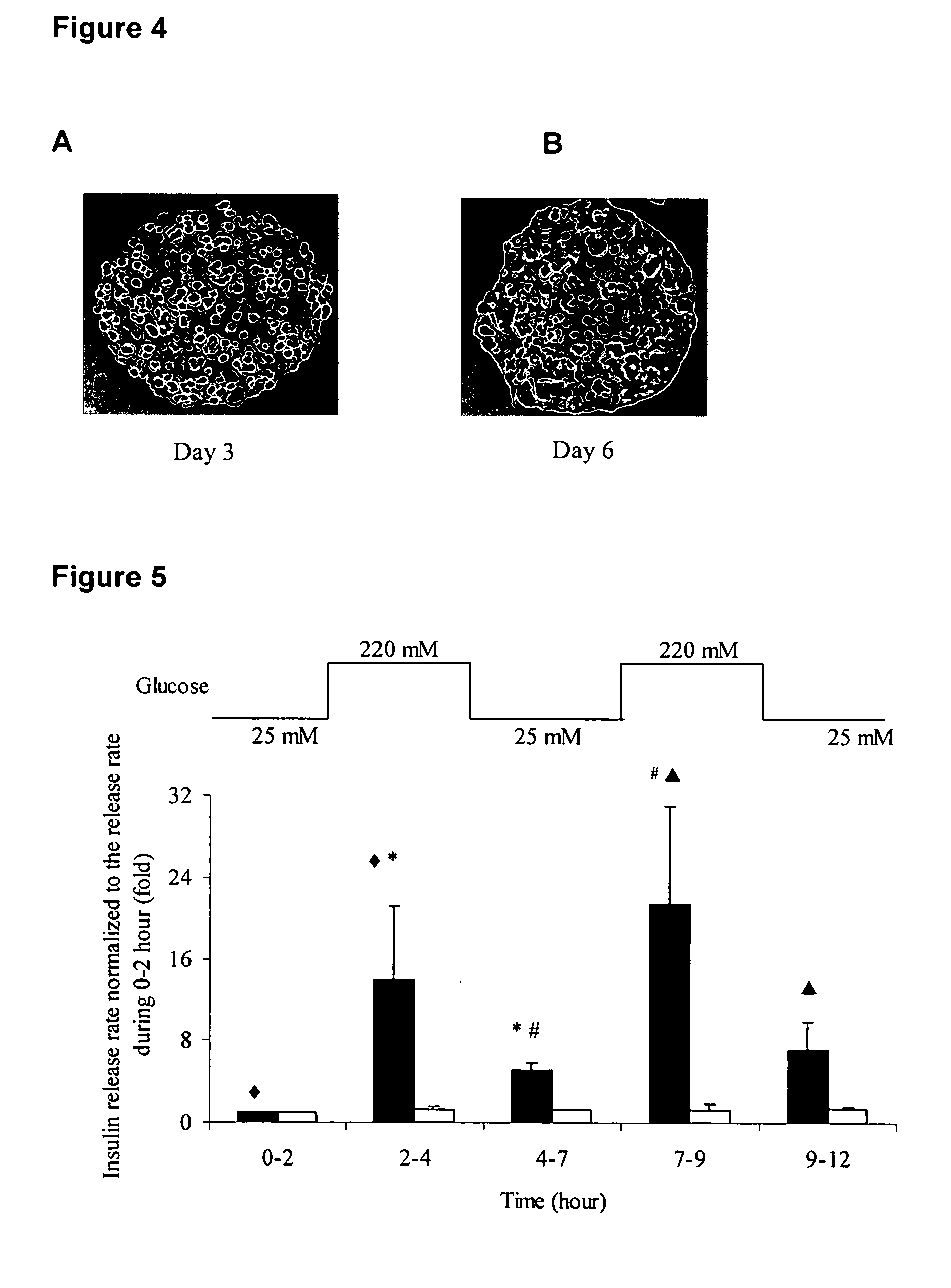
Insulin delivery system
a delivery system and insulin technology, applied in the field of insulin delivery system, can solve the problems of preventing long-term complications, providing tight glycemic regulation, and limiting the clinical application of such systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cell Culture and Transfection
[0081] Autologous non-β cells can be retrieved as a biopsy from a patient. Among non-β cells, hepatocytes and myoblasts are particularly suitable for use as β-cell surrogates. Hepatocytes can be obtained as a biopsy, they express GLUT2 and glucokinase, two major glucose-sensing components in β cells that are involved in regulating insulin secretion, and hepatic expression of insulin under transcriptional regulation has been repeatedly shown to provide some glycemic control in diabetic rodents. However, due to the absence of an acute secretory response, it is unclear that engineered hepatocytes will provide appropriate glycemic regulation in higher animals, without the risk of hypoglycemic episodes. Myoblasts also lack a regulated secretion pathway, but relative to hepatocytes they offer the advantage of easier retrievability as a biopsy, capability in amplifying into large cell number, and the ability for differentiation into stable myotubes. Moreover, ...
example 2
Cell Encapsulation
[0085] To facilitate cell loading into hybrid constructs, βTC3 cells and recombinant HepG2 and C2C12 cells were encapsulated in 2% alginate (ISP Inc., San Diego, Calif.) at a density of 7×107 cells / ml of alginate following the general protocol published by Stabler et al (Stabler et al. 2001), except that a poly-L-lysine membrane and a final alginate coating were not applied. This type of encapsulation does not introduce any significant resistance to the transport of cellular nutrients and metabolites. Briefly, cells from confluent monolayer cultures were detached by EDTA-trypsin (Sigma). A sample of the cell suspension was used for cell counting with trypan blue (Sigma); the rest of the suspension was centrifuged for 4 minutes at 110 g, and pellets were mixed with sodium alginate sterilized by filtration through a 0.2-μm syringe filter (Pall Life Sciences, Ann Arbor, Mich.). Alginate beads of approximately 700 μm diameter were formed by passing the cell-alginate s...
example 3
Characterization of Insulin Release from Constructs
[0086] Each construct was cultured for 20 hours prior to glucose concentration changes to allow insulin to accumulate inside the cell compartment. Two square-waves of glucose were implemented to test the glucose responsiveness of the hybrid construct. Immediately prior to the glucose changes, the culture medium was replaced with fresh, so when an experiment started at t=0, the surrounding medium was insulin-free and contained 25 mM of glucose. Following incubation for 2 hours in this medium, 2.0 g of glucose was directly added to the beaker to increase the glucose concentration to 220 mM. This glucose concentration is necessary to achieve transformation of the con A-based material from gel to sol (Cheng et al. 2004). For the glucose step down 2 hours later, the medium was completely replaced with fresh culture medium containing 25 mM glucose, and the cycle was repeated once more. Although higher than physiologic, use of these gluco...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar ratio | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| pore-size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com