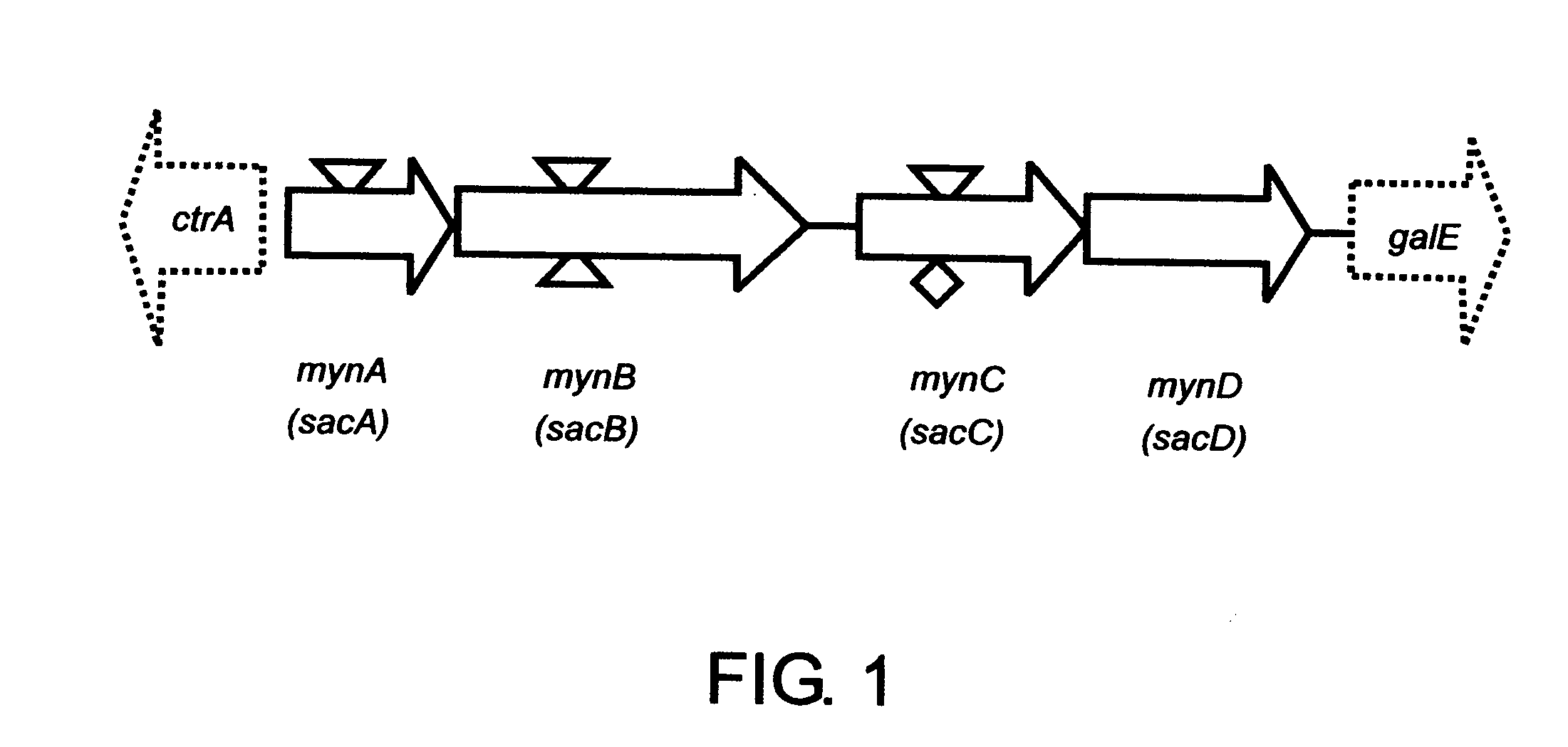
Neisseria meningitidis serogroup a capsular polysaccharide acetyltransferase, methods and compositions
a technology of capsular polysaccharide and acetyltransferase, which is applied in the field of molecular biology, can solve the problems of poor immunogenicity in young children, and achieve the effects of improving immunogenic composition, complete acetylation, and stronger immune response results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Bacterial Strains
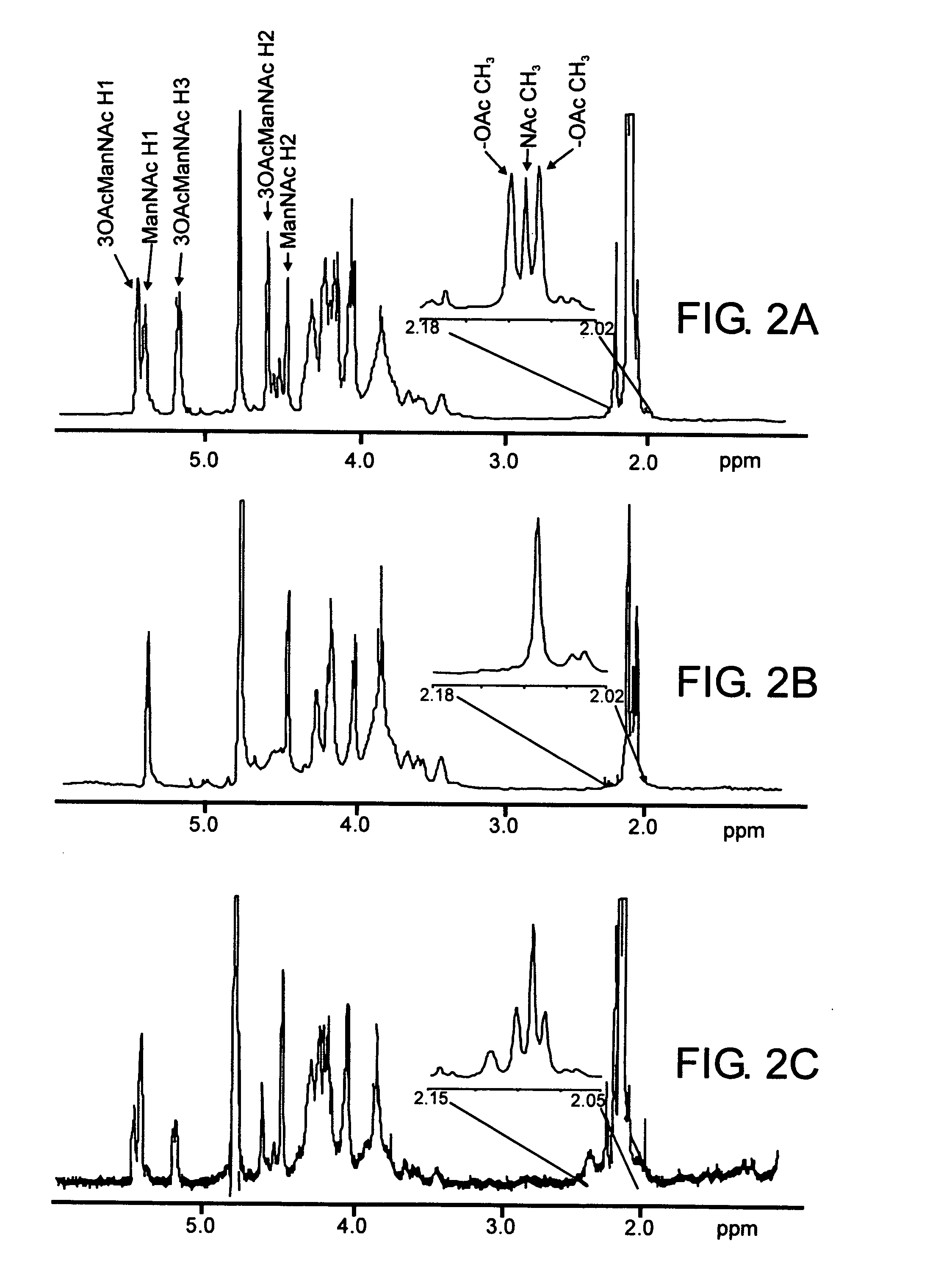
[0135] Bacterial strains, plasmids and primers used in this study are described in Table 1. The serogroup A meningococcal strains were originally isolated during an outbreak in Nairobi, Kenya in 1989 (12) and were provided by the Centers for Disease Control and Prevention (CDC), Atlanta, Ga. Strain F8229 (CDC1750) is encapsulated and was isolated from the cerebrospinal fluid of a patient with meningitis. Strain F8239 (CDC16N3) is an unencapsulated variant originally isolated as a serogroup A strain from the pharynx of an asymptomatic carrier. These strains belong to clonal group III-I and are closely related to strains that have caused epidemics in Saudi Arabia, Chad, Ethiopia and other parts of the world.
[0136] Monoclonal antibody 14-1-A (13) against meningococcal serogroup A capsular polysaccharide was provided by Dr. Wendell Zollinger, Walter Reed Army Institute of Research.
[0137] Restriction enzymes were purchased from New England Biolabs (Bever...
example 2
Growth Conditions
[0138] Meningococcal strains were grown with 3.5% CO2 at 37° C. on GC base agar (Difco, Detroit, Mich.), supplemented with 0.4% glucose and 0.68 mM Fe (NO3)3, or in GC broth containing the same supplements and 0.043% NaHCO3. BHI medium (37 g / liter brain heart infusion) with 1.25% fetal bovine serum was used when kanamycin selection was required. Antibiotic concentrations (in μg / ml) used for E. coli strains were ampicillin, 100, kanamycin, 50, and erythromycin, 300; and for N. meningitidis were kanamycin, 80, spectinomycin, 60, erythromycin, 3. E. coli DH5α strain, cultured on Luria Bertani (LB) medium, was used for cloning and propagation of plasmids. Meningococci were transformed by the procedure of Janik et al. (14). E. coli strains were transformed by electroporation (Gene-pulser Bio-Rad, Hercules, Calif., according to the manufacturer's protocol).
example 3
Construction of Meningococcal mynC Nonpolar Mutant NmA001
[0139] An internal 745-bp fragment of mynC, produced by PCR amplification using primers SE57 and SE61 (11) and the chromosomal DNA of strain F8229 as a template, was cloned into pCR2.1 to yield pGS201. The aphA-3 fragment obtained from pUC18K with EcoR I and HinC II digestion and filled in with Klenow polymerase was inserted into the unique Sspl site of mynC in pGS201 to generate pGS202. The correct orientation of aphA-3 was confirmed by colony PCR and direct sequencing analysis of pGS202. A ScaI-linearized pGS202 plasmid was used to transform serogroup A meningococcal strain F8229 to generate NmA001. The correct homologous recombination of the aphA-3 cassette into the mynC coding sequence was confirmed by PCR with cassette-specific primers and chromosomal-specific primers.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| v/v | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com