Aryl boronate functionalized polymers for treating obesity
a functionalized polymer and aryl boronate technology, applied in the field of aryl boronate functionalized polymers for treating obesity, can solve the problems of reducing patient compliance and unpleasant side effects of oil spillage from stool, and achieve the effects of reducing fat absorption, potent inhibitors of lipase enzymes, and removing fa
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
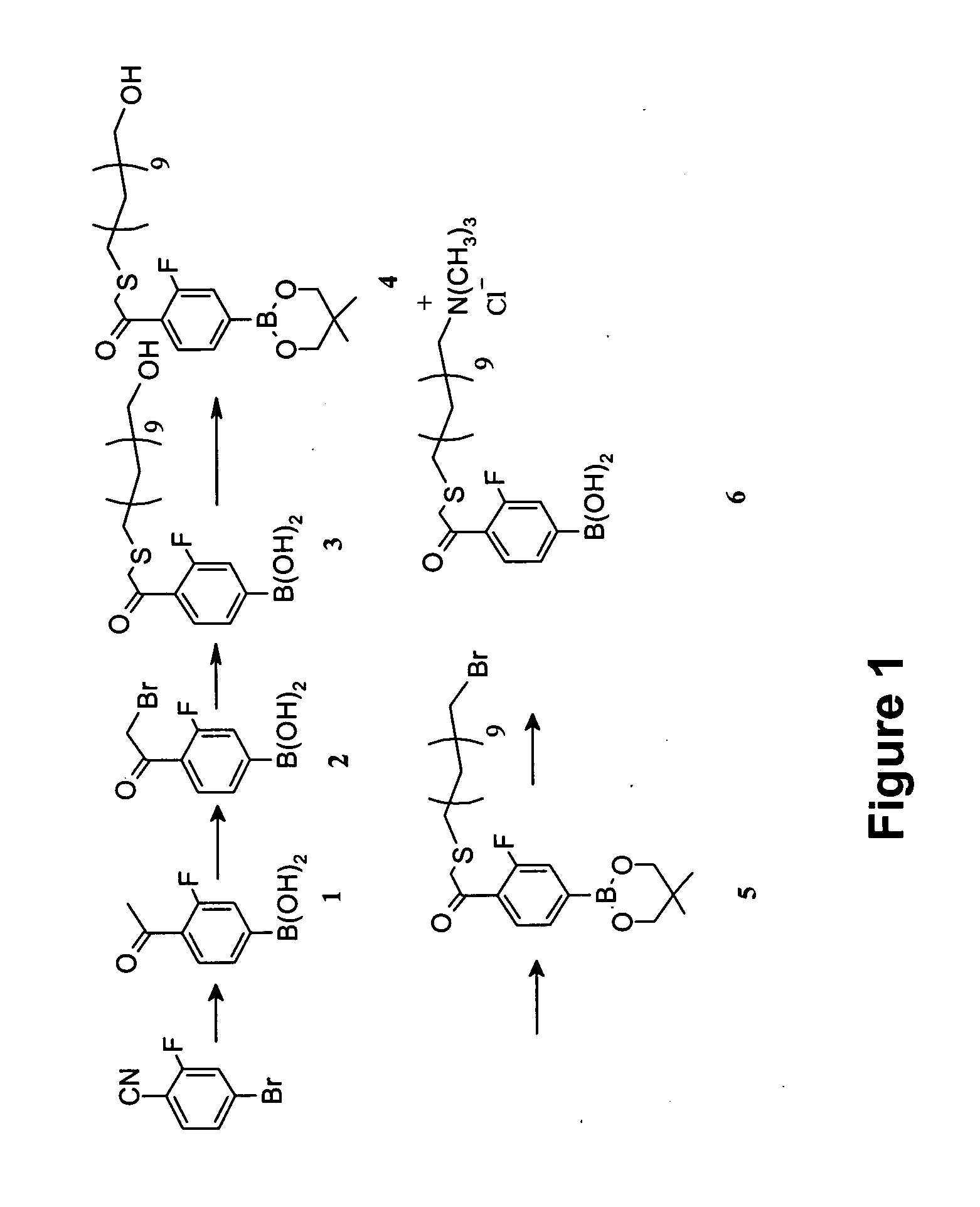
4-(14′-trimethylammonium-3′-thia-1′-ketotetradecyl)-3-fluorophenylboronic acid chloride (6)
[0087] The synthesis of Compound (6) is shown out schematically in FIG. 1. A detailed description of the procedure is provided below.
Step 1. Synthesis of 4-acetyl-3-fluorophenylboronic acid (1)
[0088] An oven-dried, 3-liter, 3-necked, round-bottomed flask (fitted with a nitrogen inlet, addition funnel, and overhead stirrer) was charged with 50 grams (0.25 mole) of 4-cyano-3-fluorophenyl bromide. Anhydrous tetrahydrofuran (200 milliliters) was added to the flask resulting in a clear solution. The solution was cooled to 0° C. using an ice bath. At this temperature, 125 milliliters of 3.0 M solution of CH3MgBr in ether (1.5 equivalents, 0.375 mole) was added slowly to the reaction flask using an addition funnel. The reaction mixture was allowed to slowly warm up to room temperature and was stirred for 48 hours. Thin layer chromatography (TLC) indicated the starting material was consumed. After ...
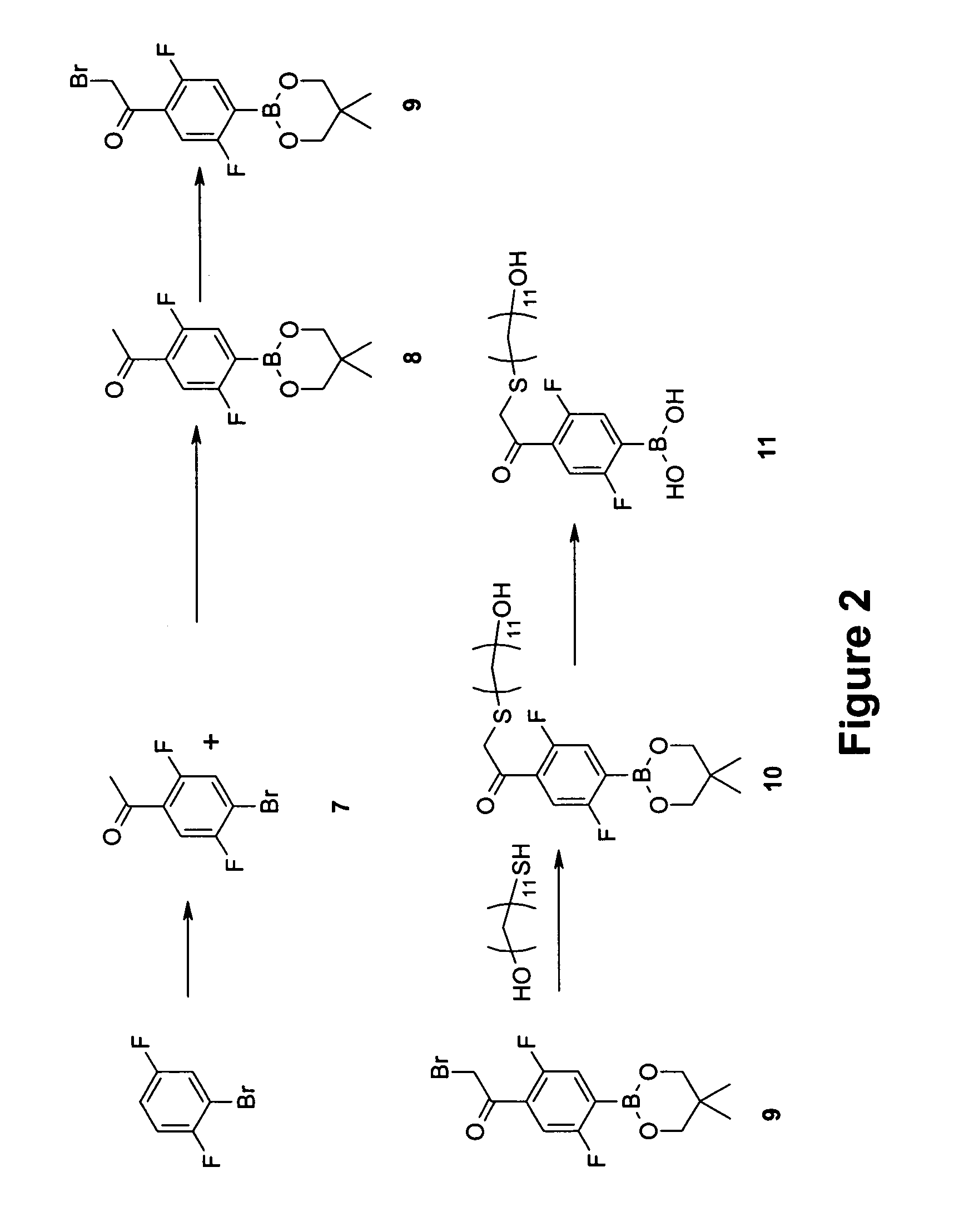
example 2
Synthesis of 2,5-difluoro-4-(14′-hydroxy-3′-thia-1′-ketotetradecyl)phenylboronic acid (11)
[0097] The synthesis of Compound (11) is shown out schematically in FIG. 2. A detailed description of the procedure is provided below.
Step 1—Synthesis of 4-bromo-2,5-difluoroacetophenone (7)
[0098] Anhydrous aluminum chloride was mixed (5 grams, 37.5 millimoles, 2.4 equivalents) with 1-bromo-2,5 difluorobenzene in a dry, round-bottom flask blanketed with nitrogen and fitted with a condenser. The mixture was heated to 60° C. and acetyl chloride (1.7 milliliters, 23.3 millimole, 1.5 equivalents) was added by syringe. The wet yellow solid changed then into a scarlet solution and was heated at 90° C. for 1 hour. The reaction mixture was poured onto 38 grams of ice, HCl was added (3 milliliters, 37% concentration) and the mixture was extracted with ether. The crude material was dried over magnesium sulfate and evaporated down. The crude material was purified by column chromatography or distilled. ...
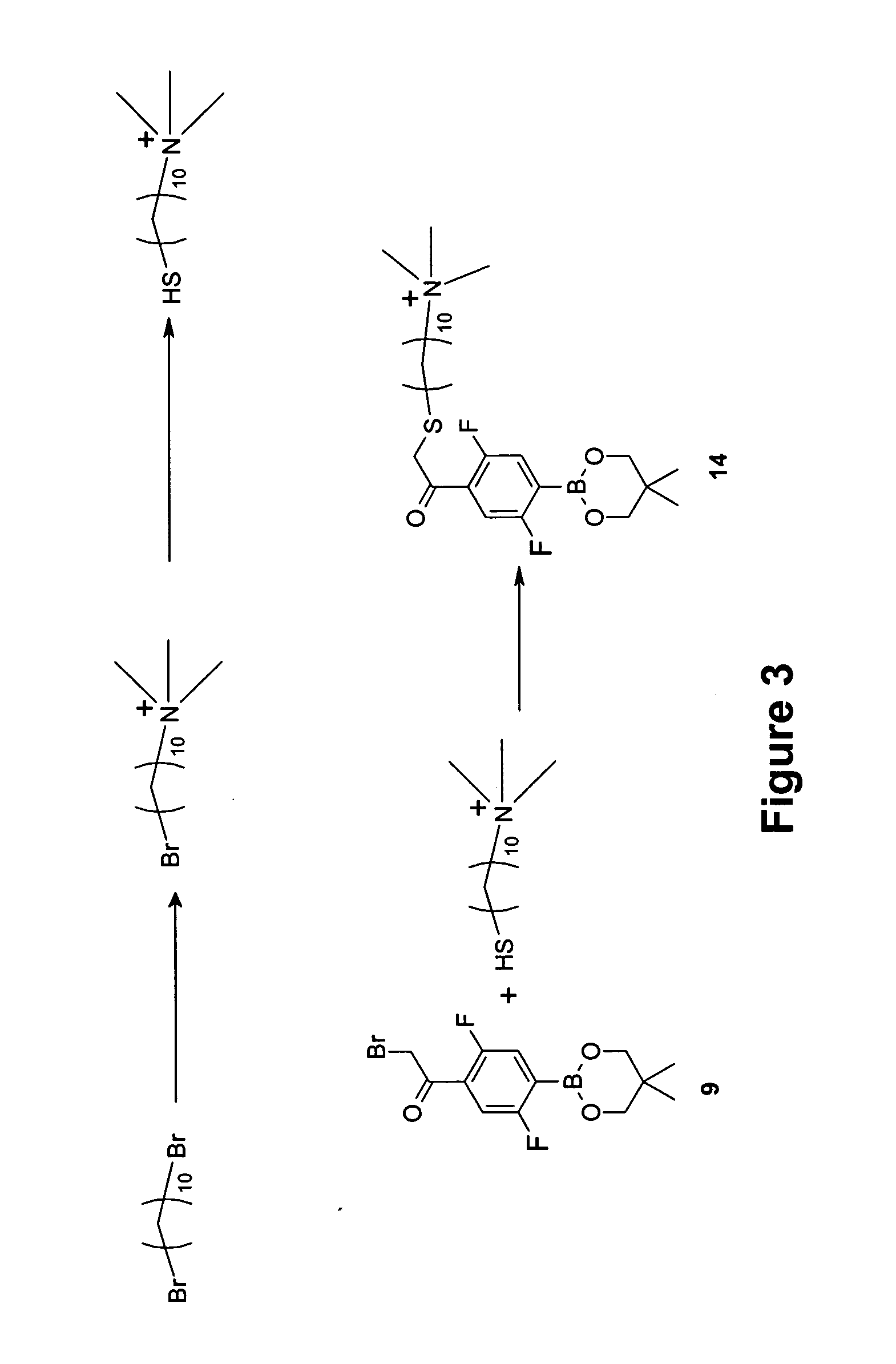
example 3
Synthesis of 2,5-difluoro-4-(13′-trimethylamonium-3′-thia-1′-ketotridecyl)phenyl(neopentyl glycolato) boron chloride (14)
[0103] The synthesis of Compound (14) is shown schematically in FIG. 3. A detailed description of the procedure is provided below.
Step 1—Synthesis of 10-bromodecyltrimethylammonium bromide
[0104] 1,10-Dibromodecane (20 grams, 66.7 mmoles) and THF (100 milliliters) were placed in a 500-mL, three-necked flask. The solution was cooled to 0° C. with an ice-water bath. Anhydrous trimethylamine (3 grams, 50.8 mmoles) was added to the mixture by slowly bubbling trimethylamine gas for about 15 minutes. Then the reaction mixture was allowed to warm to room temperature and stirred at room temperature overnight. The solid material was filtered and washed with THF (5×30 milliliters). After drying in vacuo overnight, 12.5 grams (34.82 mmoles, 69% based on the amine used) of the product was obtained as a white solid.
Step 2—Synthesis of 10-mercaptodecyltrimethylammonium bromi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com