Process for separating metals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
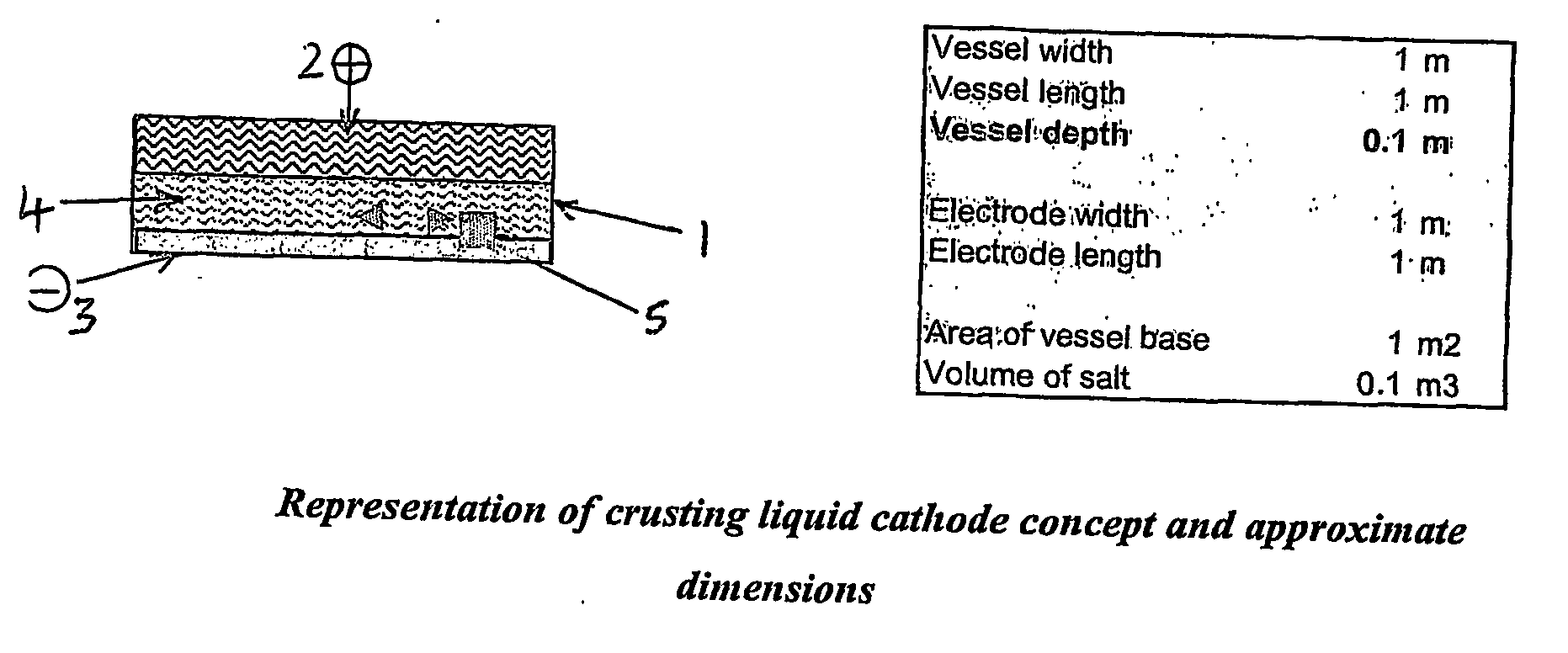
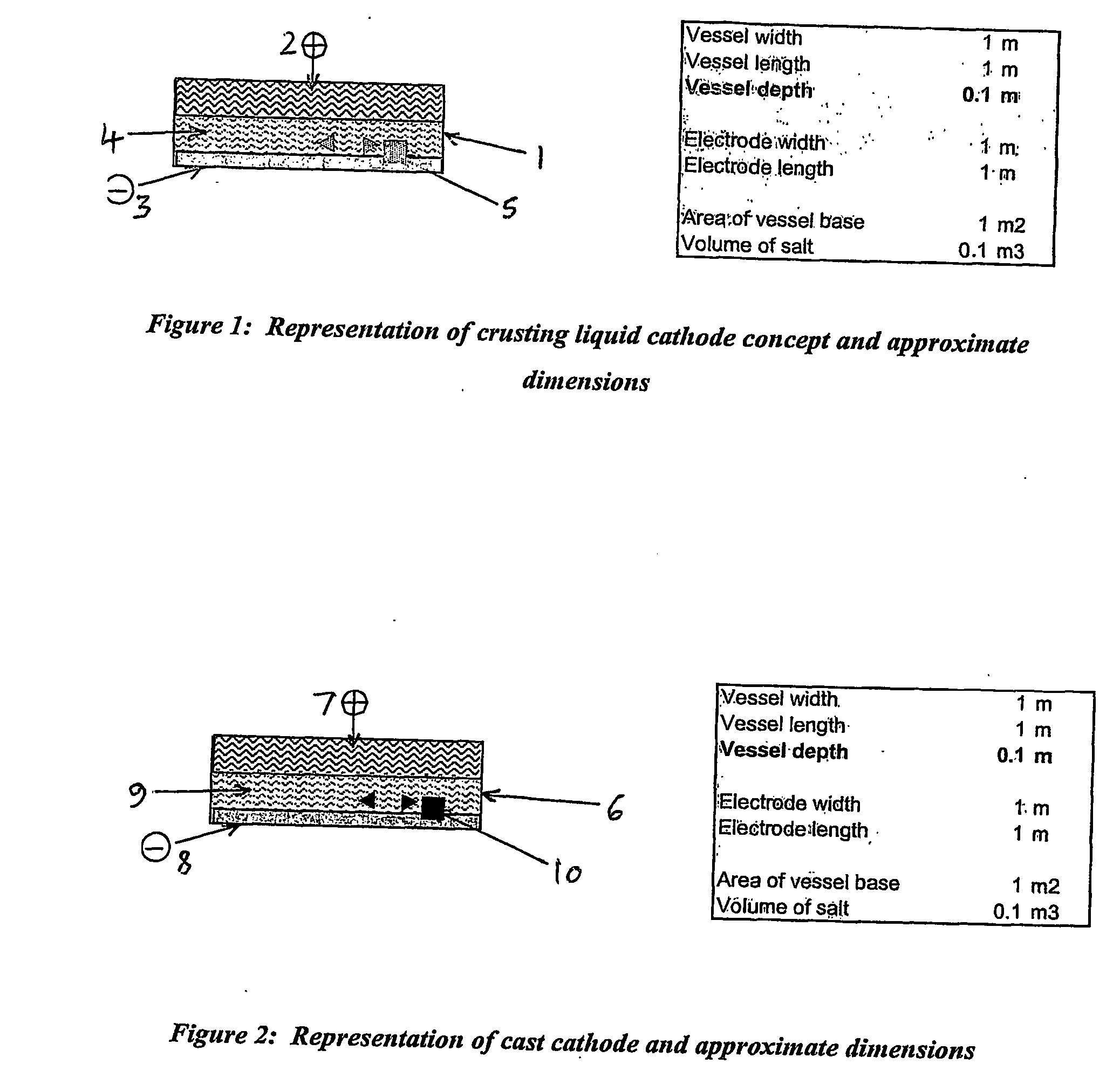
[0039] the apparatus according to the first aspect of the present invention comprises a crusting liquid cathode. Generally, said crusting liquid cathode is comprised in a horizontal thin slab configuration. Preferably, said crusting liquid cathode comprises a liquid metal cathode or liquid alloy cathode. During the electrochemical separation process for the separation of uranium metal from spent nuclear fuel, uranium metal is deposited on the cathode surface, and this metal deposit and residual amounts of cathode metal and molten salt are then removed from said cathode surface, for example by intermittent scraping or scooping. Initially, a high potential and current density may be employed in order to promote crusting of the molten metal surface by solid uranium. The metal deposit recovered at the conclusion of the process generally requires purification in order to remove contaminants, such as residual cathodic material. Typically, the anode in said apparatus comprises a basket con...
second embodiment
[0040] the apparatus according to the first aspect of the present invention comprises a cast cathode. Preferably, said cast cathode comprises a metal or alloy cathode with a lower melting point than uranium metal, the melted cathode being introduced to an electrorefiner cell as a liquid which is then frozen to form a solid metal or alloy cathode as a horizontal thin slab. In operation during the separation of uranium metal from spent nuclear fuel, uranium metal is deposited on the cathode surface, and the cathode is then melted and transferred out of the cell, transporting with it the uranium metal deposit as a slurry. The anode in said apparatus is adapted for use in a continuous process, and preferably comprises a horizontal basket containing spent fuel, which may be continuously fed and discharged transversely.
third embodiment
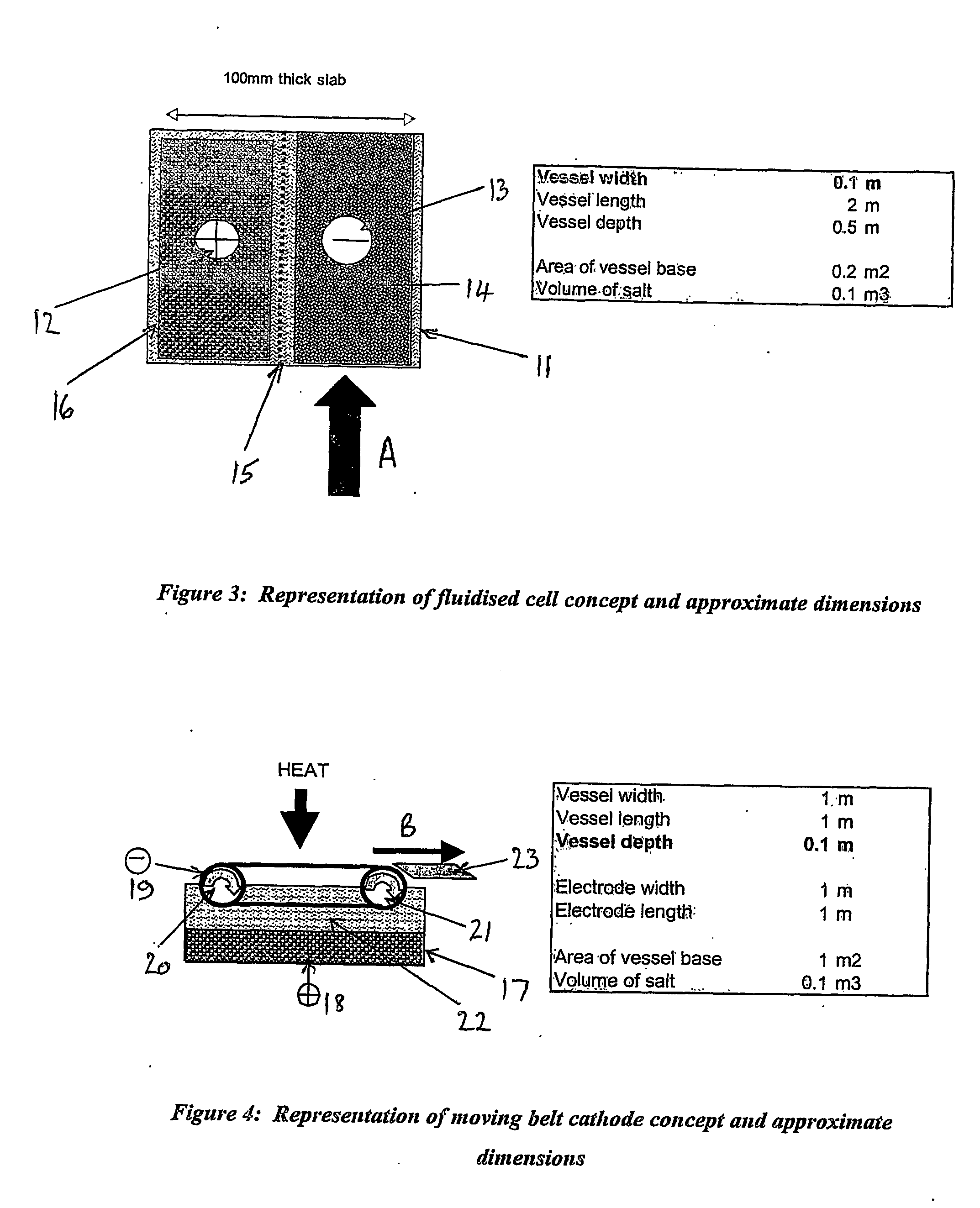
[0041] the apparatus according to the first aspect of the present invention comprises a fluidised cell or pulsed bed. Preferably said fluidised cell or pulsed bed comprises cathode beads or particulates, preferably formed from graphite or uranium, and said electrorefiner cell is divided by a ceramic non-conducting membrane, forming a vertical thin slab. In operation during the separation of uranium metal from spent nuclear fuel, a charge is applied across the anode and cathode as though parallel plates and a molten salt containing dissolved uranium ions is pumped up through the cathode bed, resulting in the formation of uranium metal deposits on the bead or particulate surface. Typically, the anode in said apparatus comprises a basket containing spent fuel, which is adapted for use in a continuous process.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Dimension | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com