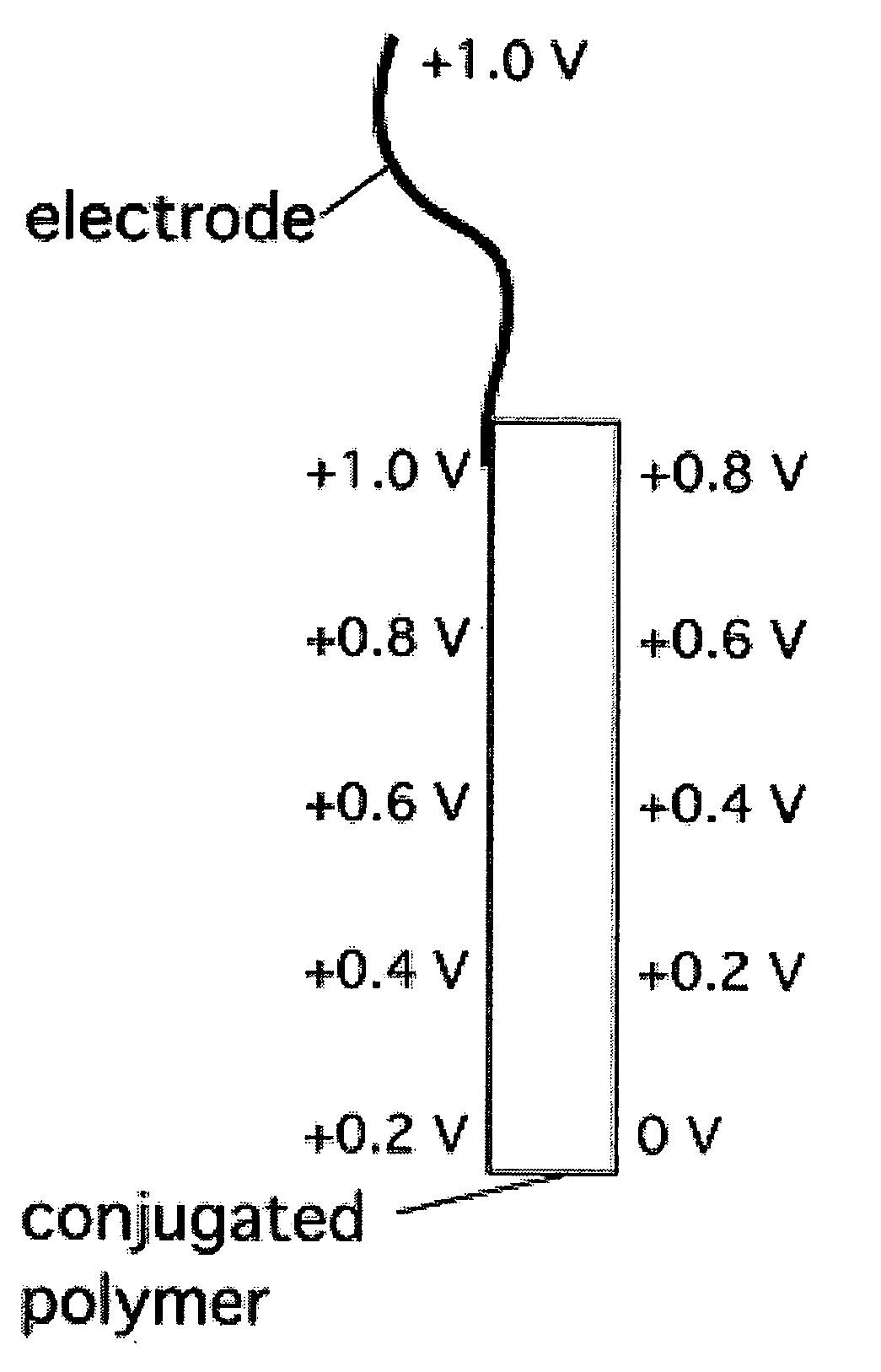
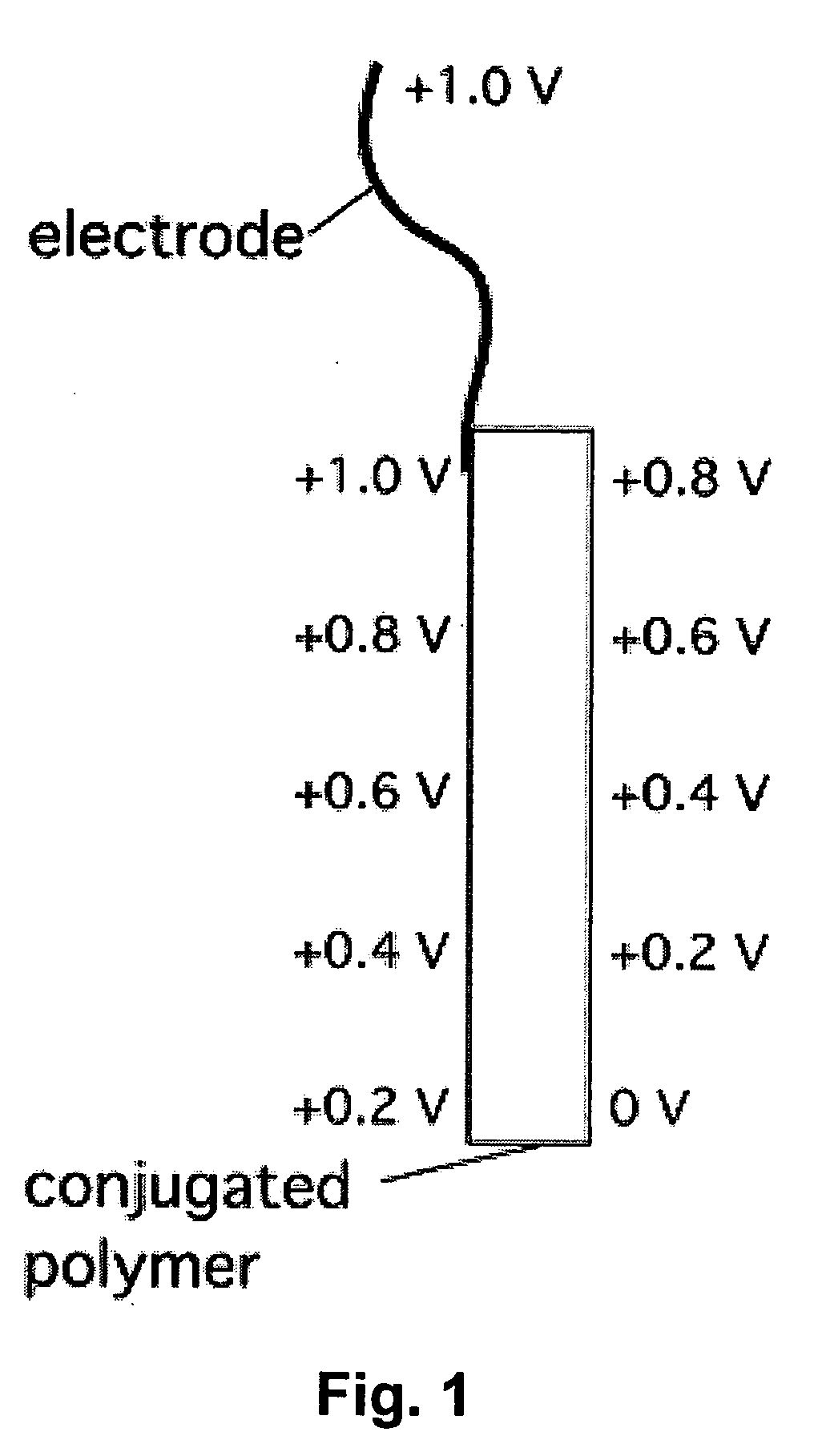
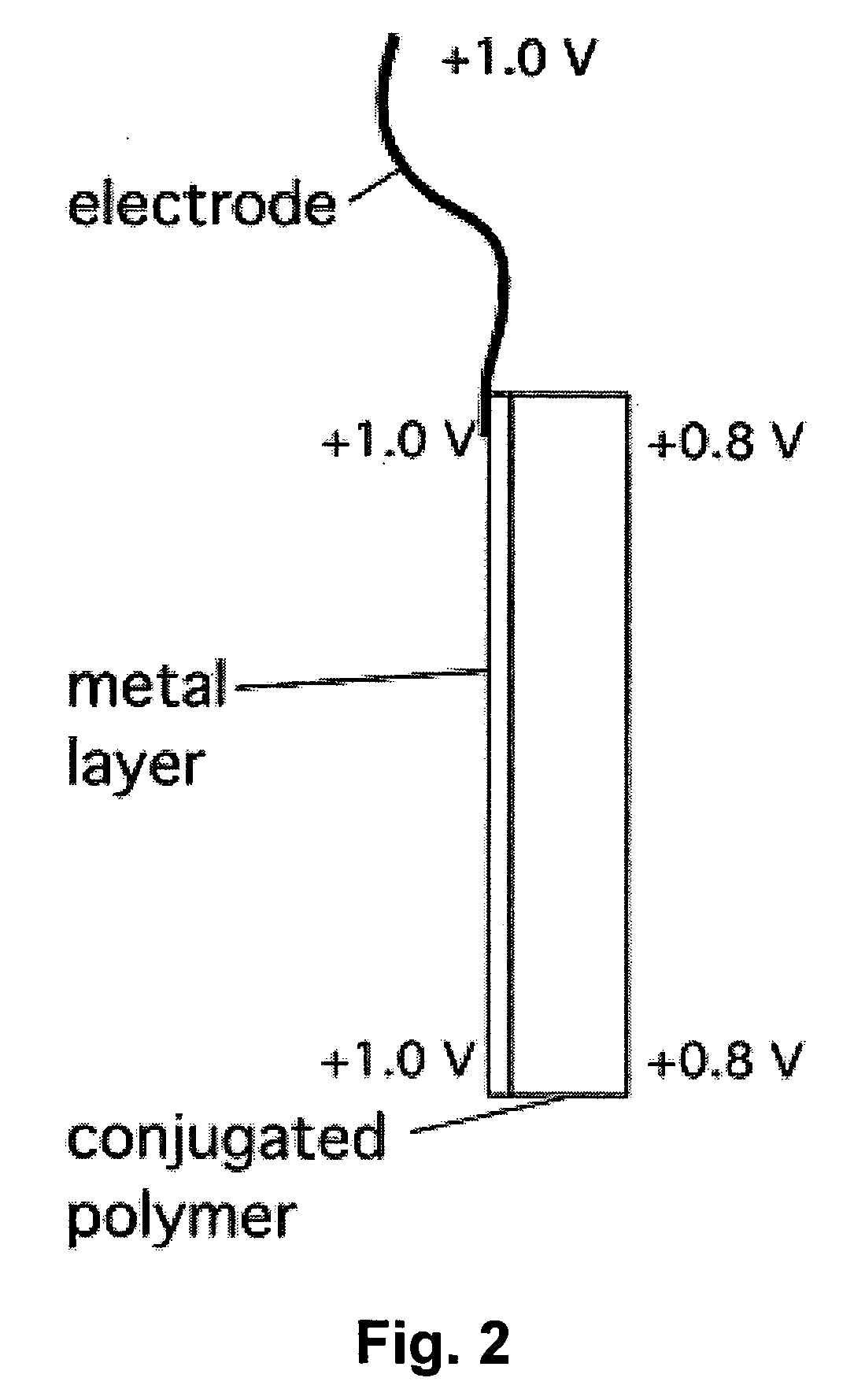
Electrochemical devices incorporating high-conductivity conjugated polymers
a technology of conjugated polymers and electrochemical devices, which is applied in the direction of non-metal conductors, conductors, machines/engines, etc., can solve the problems of inability to achieve complete and timely oxidation/reduction, material instability in air atmosphere, and film resistance, etc., to achieve low voltage operation, short response of actuators, and light weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A. Cyclic Voltammetry of PANI / AMPSA in 1 M HCl:
[0064] A PANI / AMPSA film (high conductivity material) with a thickness of approximately 15 μm was produced using the method described above. The stretched film was cut into strips approximately 1-2 mm wide and 3 cm long. The strip was immersed into a 1 M HCl aqueous solution and clamped at the bottom and affixed to the mechanical measurement arm as described above. Cyclic voltammograms were recorded by a potentiostat between −0.25 and +0.5 V. The scan rate was 50 mV / s. FIGS. 5 through 8 compare the cyclic voltammograms of low-conductivity polyaniline (˜30 S / cm) and high-conductivity polyaniline / AMPSA (˜400 S / cm) films in 1M HCl. In FIG. 5, the two oxidation peaks show the conversion of the leucoemeraldine to the emeraldine state and the conversion from the emeraldine to the pernigraniline state. The two reduction peaks show the conversion from the pernigraniline to the emeraldine state and the conversion from the emeraldine state to t...
example 2
Volume Change of PANI / AMPSA in 1 M HCl:
[0068] Without electrochemical oxidation / reduction reactions, no volume change occurs in conjugated polymers. This will be further illustrated in the examples below. With oxidation / reduction, volume change can occur. A strip of stretched PANI / AMPSA prepared as described above was cut into a strip 2 mm×3 cm. It was affixed to the mechanical measurement arm as described above. Approximately 1 cm of the strip was immersed in a 1 M HCl electrolyte. Cyclic voltammograms were recorded between −0.2 and 1 V (vs. Ag / AgCl) at a scan rate of 50 mV / s. A constant 5 g force was applied to the strip, and the change in elongation was measured. The high conductivity PANI / AMPSA underwent lineal extension / contraction, as shown in FIG. 9. The film elongated during the first oxidation process, in which the film went from the leucoemeraldine to the emeraldine state. It contracted during the second oxidation process, in which it went to the pernigraniline state. Th...
example 3
A. Cyclic Voltammetry and Linear Extension of PANI / AMPSA in 1 M LiClO4 in PC:
[0069] A strip of stretched PANI / AMPSA film prepared as described above was cut into a strip 1 mm×3 cm. It was affixed to the mechanical measurement arm as described above. Approximately 1 cm of the strip was immersed in a 1 M LiClO4 / PC electrolyte. Cyclic voltammograms were recorded between −0.6 and 1 V (vs. Ag / Ag+) at a scan rate of 5 mV / s. A constant 2.5 g force was applied to the strip, and the change in elongation was measured. Cyclic voltammograms and length change are shown in FIG. 10 and FIG. 11. The electrochemical oxidation and reduction of polyaniline is accompanied by ion transport that maintains charge neutrality. AMPSA is insoluble in PC, so it does not exit the polymer when reducing potentials are applied. This prevents the reduction reaction from taking place. This is shown in FIG. 10: there is almost no current recorded during the cyclic voltammogram. There is also essentially no length c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com