Electrochemical device
a technology of electrochemical devices and electrodes, which is applied in the direction of non-aqueous electrolyte cells, cell components, electrochemical generators, etc., can solve the problems of deteriorating charge-discharge cycle characteristics of secondary batteries, impaired incombustibility of nonaqueous electrolyte, etc., and achieve excellent charge-discharge cycle characteristics and incombustibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
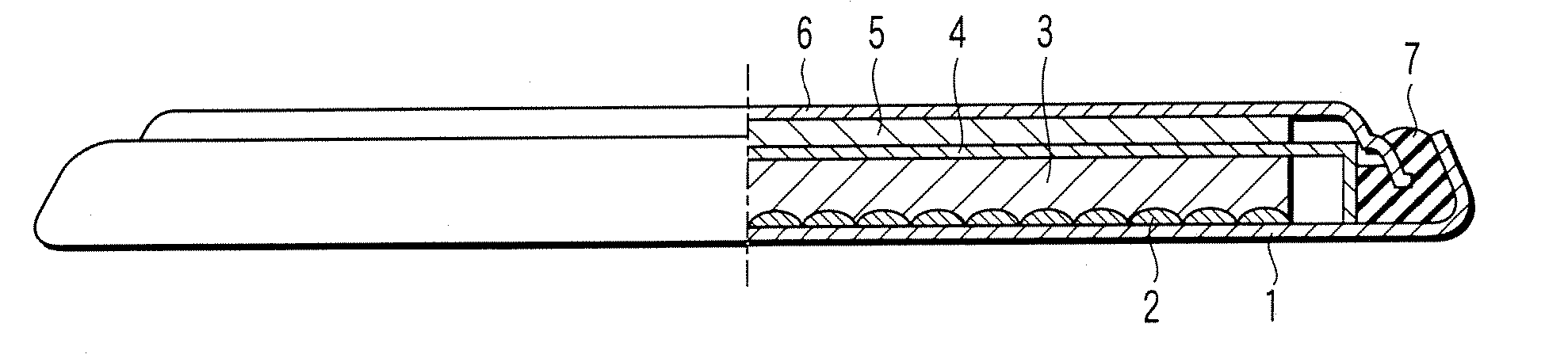
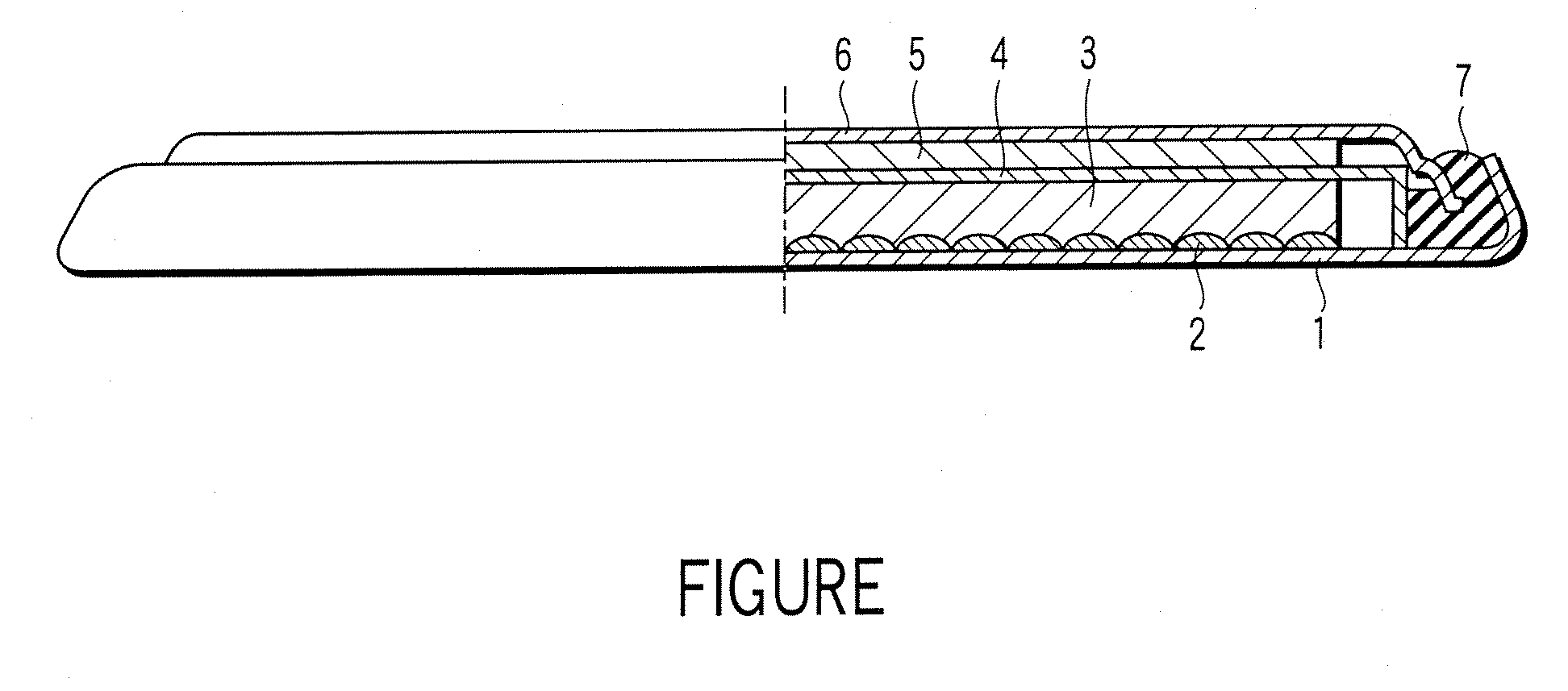
[0025] A lithium ion secondary battery according to a first embodiment of the present invention will now be described with reference to FIGURE. Specifically, FIGURE is a cross sectional view, partly broken away, schematically showing the construction of a lithium ion secondary battery according to the first embodiment of the present invention. Incidentally, the lithium ion secondary battery showing in FIGURE is in coin shape.
[0026] As shown in the drawing, the lithium ion secondary battery comprises a case 1. A positive electrode current collector 2 is placed on the inner bottom of the case 1. Also, a positive electrode 3 is housed in the case 1. The positive electrode 3 is electrically connected to the case 1 via the positive electrode current collector 2. On the other hand, a negative electrode 5 is electrically connected to a negative electrode sealing plate 6 that also acts as a negative electrode current collector. The negative electrode sealing plate 6 is fixed to an opening ...
second embodiment
[0099] An electric double layer capacitor according to a second embodiment of the present invention will now be described with reference to FIGURE. Incidentally, the following description covers only the portion differing from the first embodiment.
[0100] The electric double layer capacitor has a construction similar to that of the lithium ion secondary battery shown in FIGURE. For example, in the case of the electric double layer capacitor, each of the positive electrode 3 and the negative electrode 5 corresponds to a polarizable electrode. In view of the aspect of improving the energy density, it is desirable for the polarizable electrodes respectively corresponding to the positive electrode 3 and the negative electrode 5 to have substantially the same volume. Incidentally, in the case of the electric double layer capacitor, each of the positive electrode current collector 2 and the negative electrode current collector 6 corresponds to a collection electrode.
[0101] Each of the po...
example 1
[0104] Reaction was carried out at room temperature for 6 hours between 4-chloro-1,3-dioxolan-2-one (compound A) and dimethyl ethyl amine (compound B) within acetonitrile. Then, acetonitrile was evaporated until the volume of the reaction mixture was decreased to ½ the original volume. Further, ethyl acetate was added to the reaction mixture, followed by filtering the resultant precipitate and subsequently drying the precipitate under a reduced pressure. In the next step, reaction was carried out at room temperature for 6 hours between the powder obtained by the drying under the reduced pressure and LiTFSI (compound C) within acetonitrile. The resultant precipitate was removed, and the acetonitrile solution was evaporated under a reduced pressure so as to obtain a room temperature molten salt represented by formula (11) given below.
[0105] Incidentally, it is possible to decrease the amount of the impurities contained in the room temperature molten salt by using AgTFSI in place of L...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| interplanar spacing d002 | aaaaa | aaaaa |
| interplanar spacing d002 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com