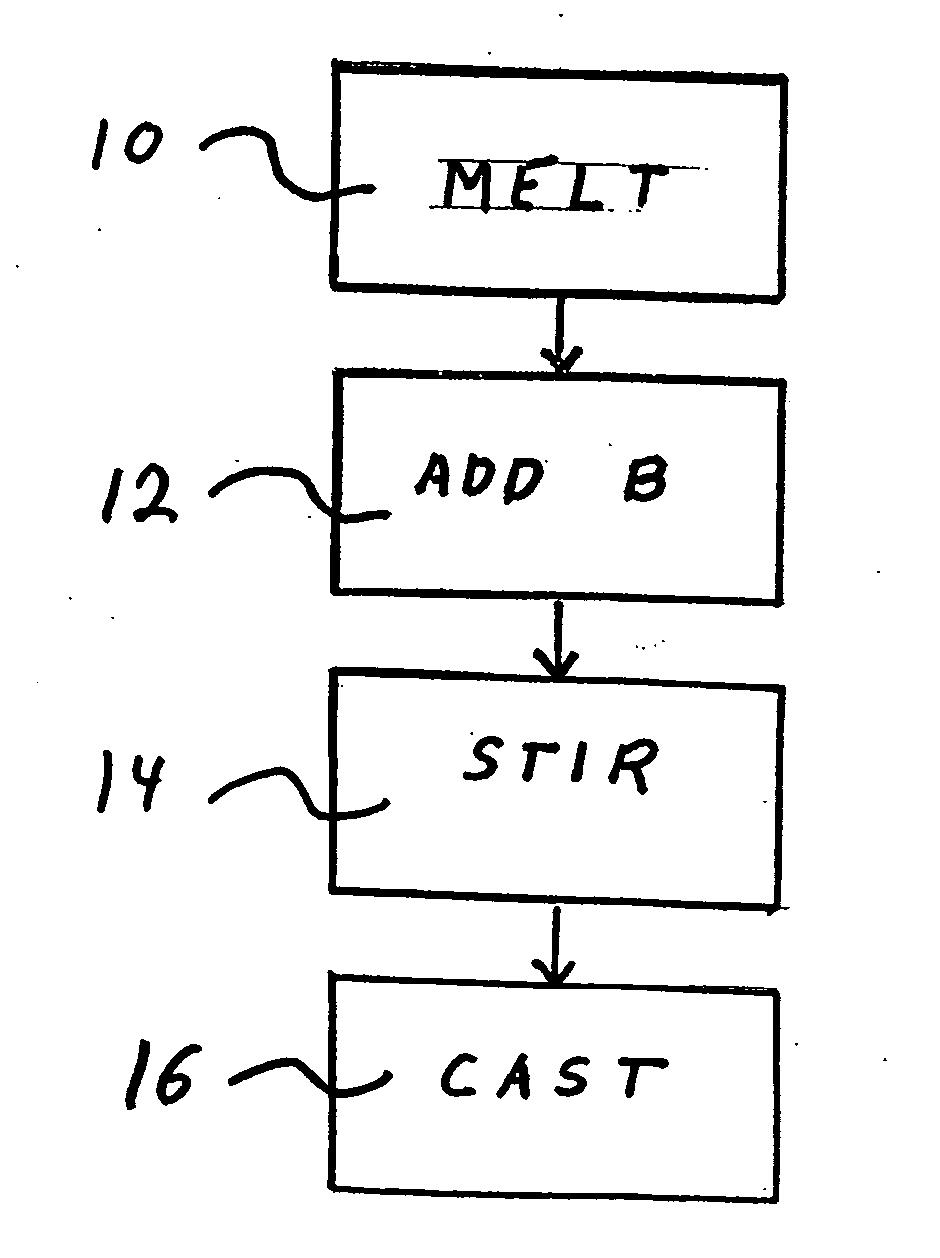
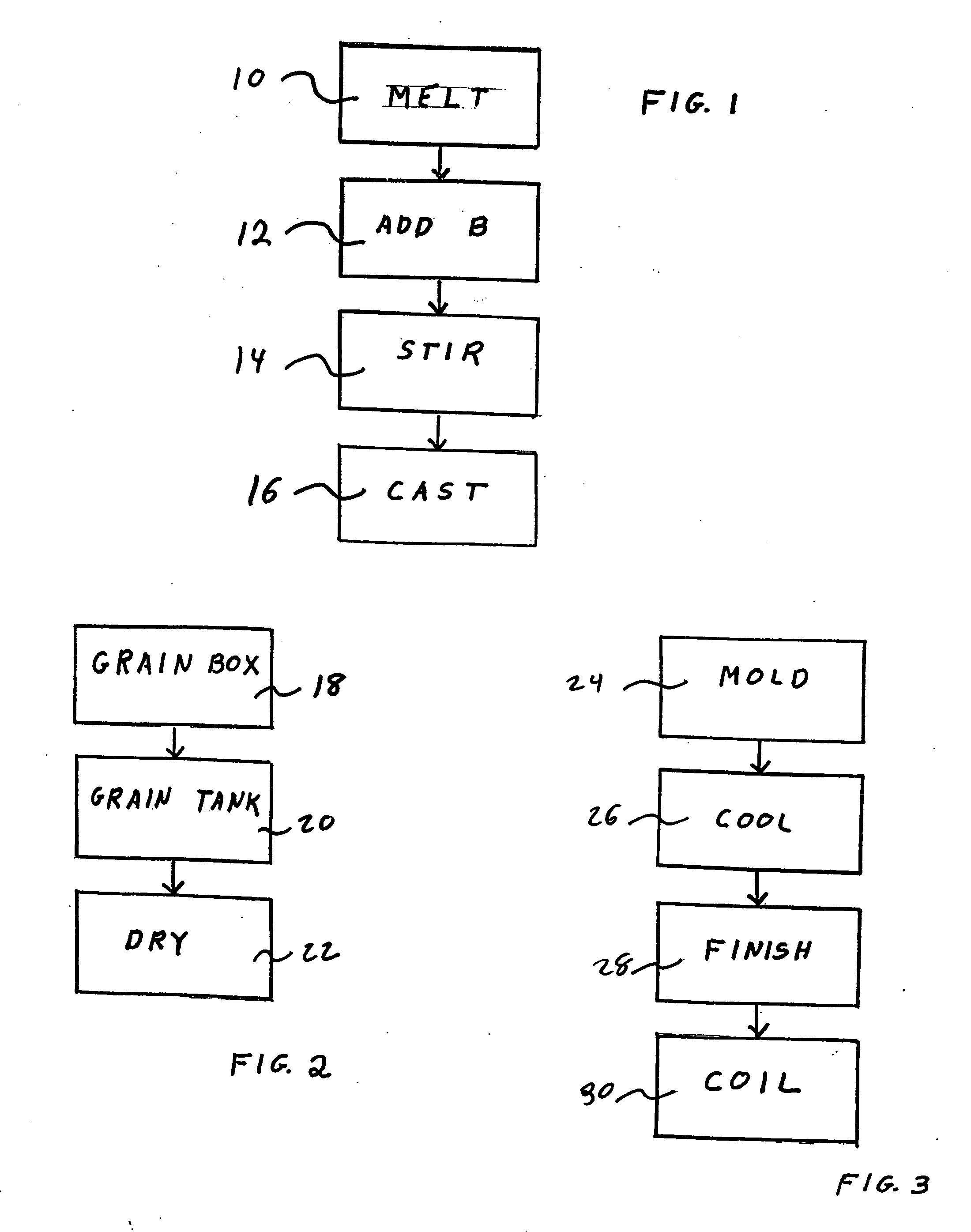
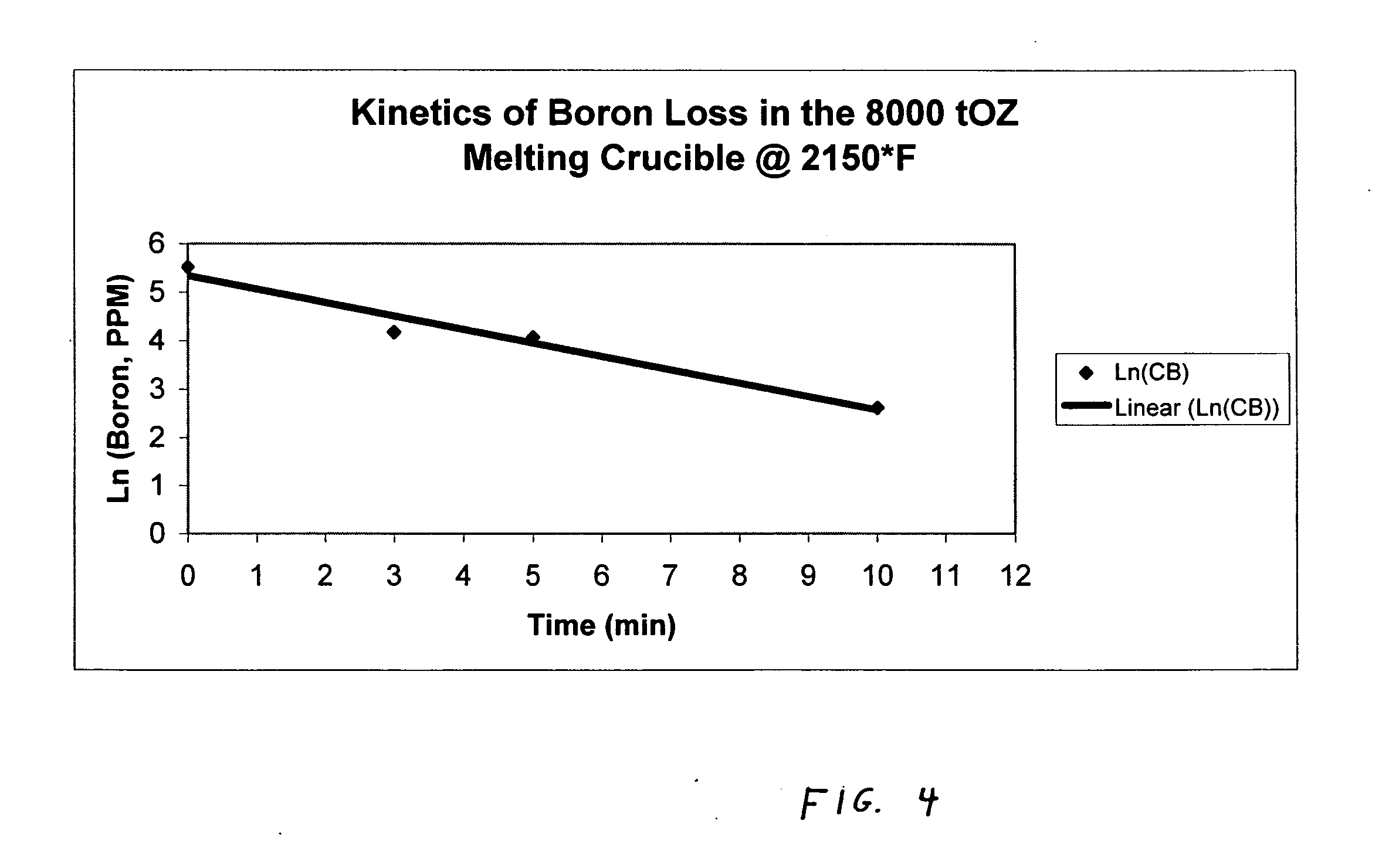
Method for adding boron to metal alloys
a metal alloy and boron technology, applied in the field of adding boron to metal alloys, can solve the problems of frequent introduction of hard spots into products, needless expense in the processing of ultimately unsatisfactory products, and frequent contamination of 2% boron master alloys with silicon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(Prophetic)—Ag—Cu—Ge—Si Alloy
[0061] A silver alloy is made by melting together 93.2 weight percent fine silver casting grains, 1.3 weight percent germanium in the form of small broken pieces, 0.2 weight percent silicon (added as a Cu / Si master alloy containing 10 weight percent silicon) and the balance being copper granules. Melting is by means of a gas-fired furnace heated to a pour temperature of about 2000° F. The melt is covered with graphite to protect against atmospheric oxidation. In addition, a hydrogen gas protective flame is provided. Stirring is by hand using a graphite stirring rod.
[0062] When the alloy constituents liquefy, 20.2 grams (0.65 troy ounce) of sodium borohydride per 46.7 kilogram (1500 oz.) melt are wrapped in a pure silver foil, about 0.15 mm thick. The foil wrapper holds the sodium borohydride to prevent it from floating to the surface of the melt. The wrapped sodium borohydride is placed into a hollow cup-shaped end of a graphite stirring rod and plunge...
example 2
(Working)—Manufacture of Sterling Silver Casting Grain
[0065] Two hundred troy ounces of a sterling silver precursor melt were melted in a clay-graphite crucible. The precursor melt had a nominal composition, by weight, of 93% silver, 5.7% copper and 1.3% germanium. The precursor melt constituents were mixed together and heated under a carbon monoxide flame and covered with a one inch thick layer of borax salt. When the precursor melt temperature reached the flow temperature, 0.0125% boron was added as NaBH4. The boron compound was wrapped in 0.15 mm silver foil for introduction to the master melt. Sufficient power was provided to maintain the temperature of the molten precious metal alloy at the flow temperature. The molten precious metal alloy was then stirred with a graphite stirring rod for 3.7 minutes and poured into a grain box. The molten precious metal alloy was protected by a reducing atmosphere during pouring at the flow temperature. After about 0.25 minutes, the entire mo...
example 3
(Working)—Manufacture of Sterling Silver Grains by Batch Process
[0067] Table 1 illustrates that the process of the invention is effective to add boron to a sterling silver precursor alloy and that the melt cover appears to have more of an effect on the boron content in the precious metal alloy than does the pour gas. In no instance were hard spots detected on the cast grains.
TABLE 1BoronTimeContentBetweenBoronAmountinBoronContentMeltNaBH4PourPrecursorAdditionin CastMeltSizeAddedPourMeltTemp.Alloyand PourGrainsNumber(troyoz)(grams)GasCover(° F.)(ppm)(minutes)(ppm)02812002.78N2 / H2Borax21501253.713.8032215003.33N2 / H2Borax2150204.09.503262003.04COBorax21501374.2523.503272003.04COBorax21501376.56.10338 / 34002.02COBorax215045.55.023.9035315005.05N2 / H2Borax215030.35.2512.20339728.09COBorax215010113.09.0081006 3003.03COBorax2150914.011.4B0120017.77COGraphite20008005.00.6B022008.89COGraphite20004005.00.6B032005.55COGraphite20002505.01.3BS420035.53COGraphite220016006.42.4BS052008.89COGraphi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| melting temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com