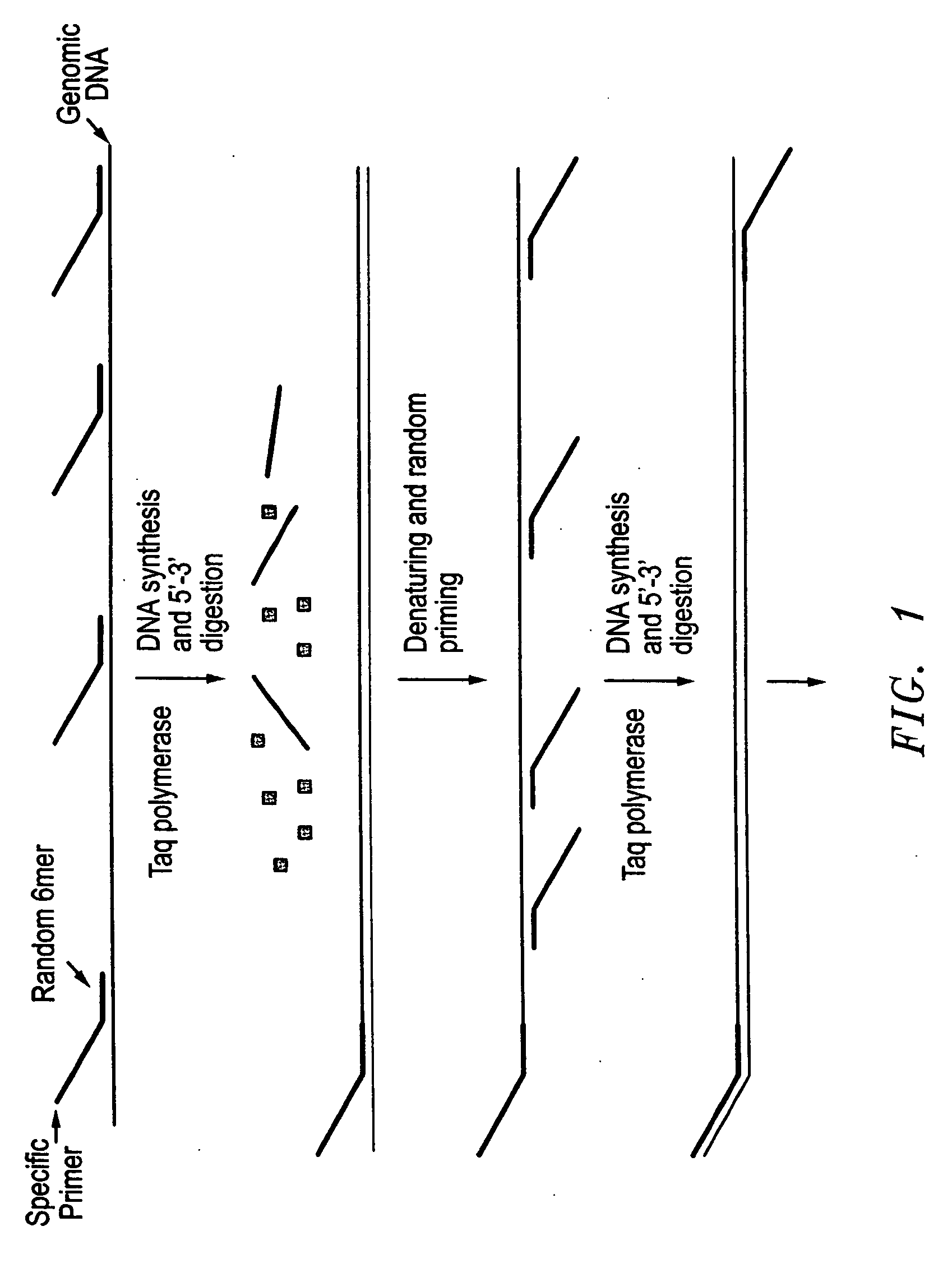
Method for rapid amplification of DNA
a dna amplification and rapid technology, applied in the field of dna amplification, can solve the problems of limiting the usefulness of the information obtained from the dna sample, and achieve the effects of reducing the risk of sample contamination, facilitating high-throughput screening, and overcoming inherent drawbacks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0203] The efficiency of PCR™ amplification of DNA samples stored on FTA paper processed using the presently disclosed precipitation method and the commercially produced FTA Purification Reagent (manufactured by Whatman) protocol was compared. First, four bloodstain DNA samples stored on FTA paper were processed using the disclosed precipitation method according to the following protocol: A small circle (1-3 mm) in the FTA paper sample was excised by the commercial Harris Micro-Punch manufactured by Shunderson Communication, Ottawa, Ontario, Canada, and washed with distilled water. The circle was transferred to a 96-well plate and soaked in 200 μl of DD H2O for 20 minutes. The water was changed and the circle was soaked in water again for 5 minutes. The water was next replaced with 200 μl of 0.3M NaOAc / Ethanol (50 / 50 v / v) solution and the paper was soaked for 5 minutes to fix the genomic DNA on the paper. The solution was removed and the paper was washed in 200 μl of 80% ethanol for...
example 2
[0207] The method of amplifying whole genomic DNA of the present disclosure was used to amplify genomic DNA from bovine bloodstain samples stored on FTA paper. This method combines DNA extraction and amplification in a single operation by allowing genomic DNA in a bovine bloodstain sample to be amplified in a single reaction mixture using a single thermocycling reaction. Therefore, this method greatly reduces the risk of sample contamination and facilitates high-throughput screening. Additionally, 96-well or 384-well plates can be utilized for amplification of genomic DNA stored on ETA paper using the presently disclosed methods, which greatly facilitates a high-throughput operation. The utility and efficiency of the presently disclosed method of DNA amplification was tested by comparing PCR amplification of a known bovine SNP locus using DNA amplified by the disclosed method and DNA bound directly to FTA paper. In both experiments, the DNA samples stored on FTA paper were processed...
example 3
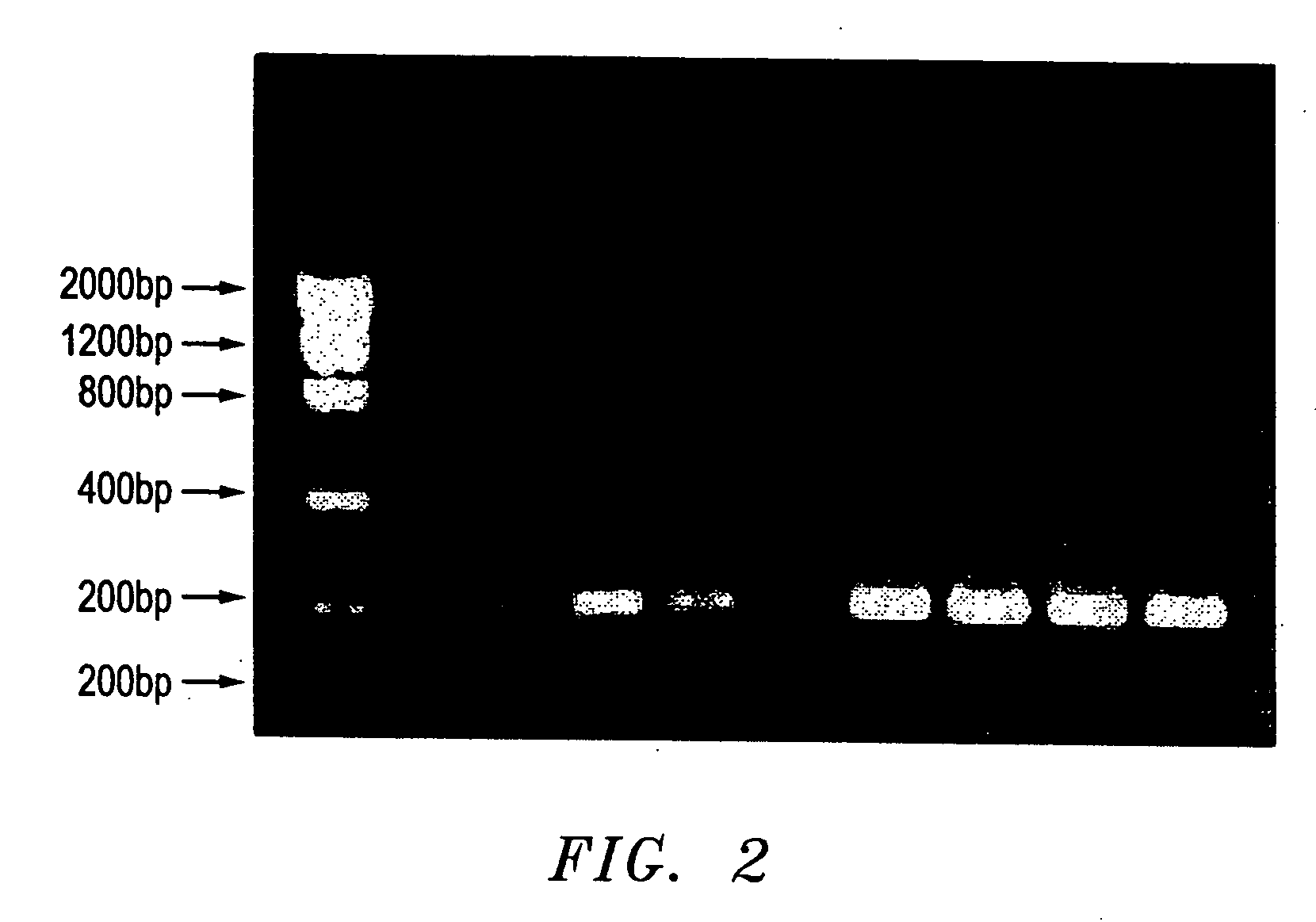
[0213] To compare the efficiency of the presently disclosed method of DNA amplification, DNA samples stored on FTA paper were first processed using the presently disclosed precipitation method or the commercial FTA Purification Reagent protocol, and then amplified according to the disclosed DNA amplification method. Two sets of punches from six different bloodstains stored on FTA paper were first treated using either the disclosed precipitation method or the FTA Purification Reagent protocol as outlined in Example 1. Next, the two sets of DNA samples were amplified according to the disclosed DNA amplification method, as outlined in Example 2.
[0214]FIG. 4. demonstrates the results of genomic DNA amplification using the disclosed method of DNA amplification. The figure compares the efficiency of the disclosed DNA amplification method when the DNA sample is processed using the FTA Purification Reagent protocol (lanes 2-7) versus the disclosed precipitation method (lanes 8-13). FIG. 4....
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com