Process for reforming hydrocarbons with carbon dioxide by the use of a selectively permeable membrane reactor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
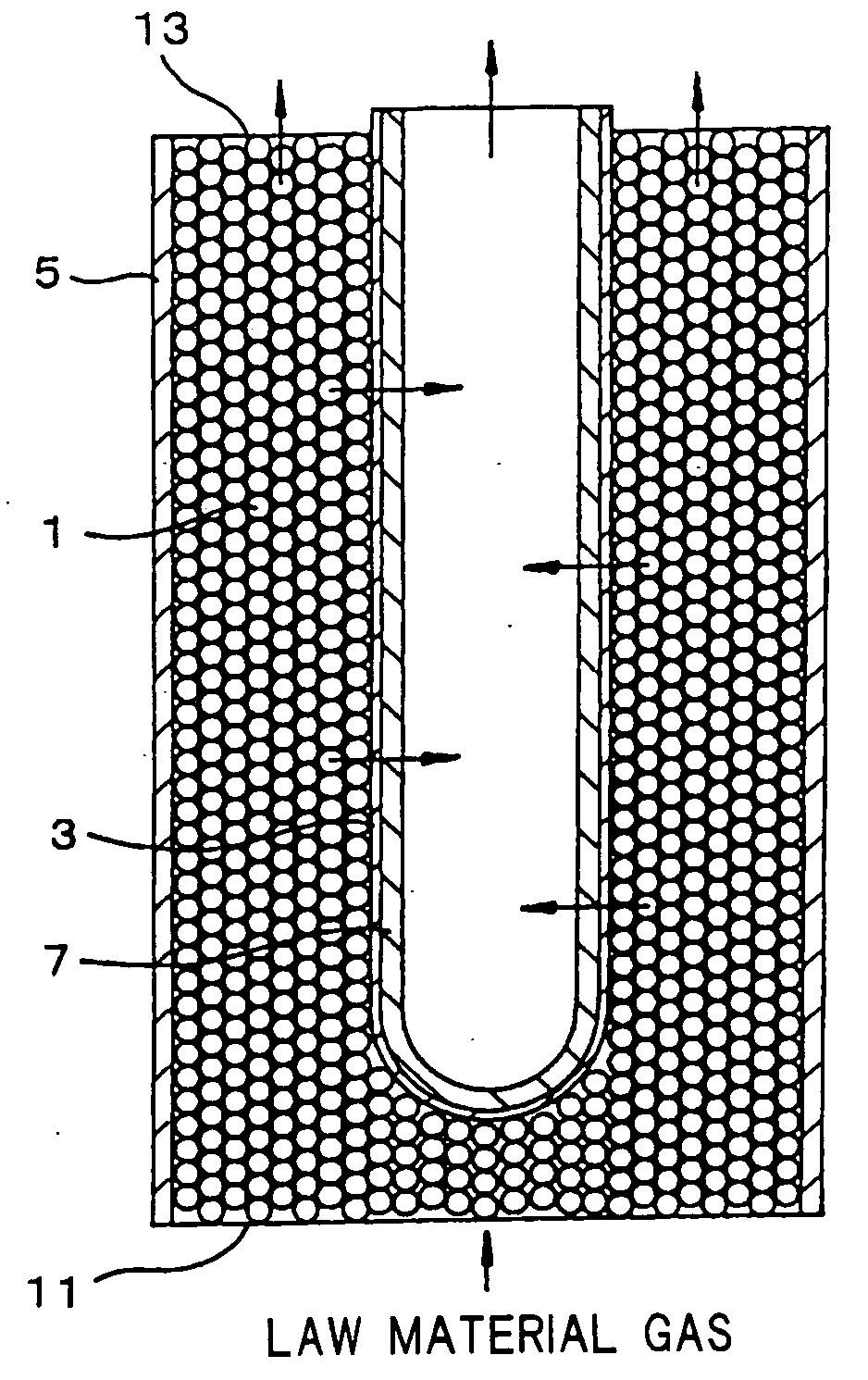
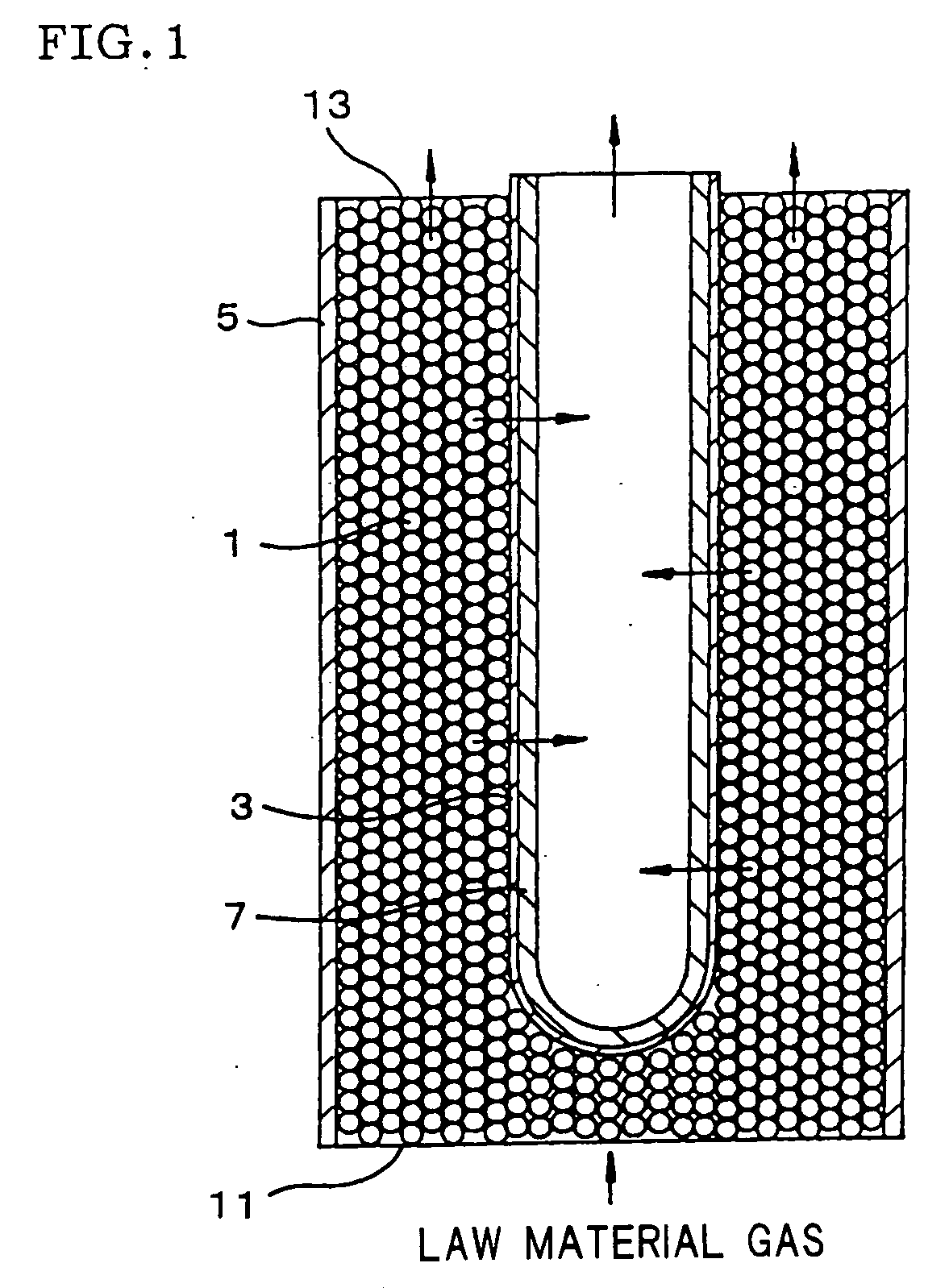
[0028] A selectively permeable membrane reactor having a structure as shown in FIG. 1 was produced using a palladium membrane as the selectively permeable membrane (hydrogen permeable membrane) and a catalyst in which platinum was supported on the surface of a zirconia carrier in the shape of pellets. A raw material gas containing methane, carbon dioxide, and steam was supplied to the selectively permeable membrane reactor at a molar ratio of CO2 / CH4=1.0 and H2O / CO2=0.05 to effect a carbon dioxide reforming reaction of methane and a reaction occurring along with the reforming reaction to separate hydrogen from the reaction products. The reaction temperature was 550° C., the reaction-side pressure was 1 atm (101.3 kPa), and the permeation-side pressure was 0.1 atm (10.1 kPa). The flow rate of the raw material gas was adjusted so that the flow rate of methane was 100 ml / min. The conversion rate of methane was measured in the initial stage of the reaction (30 minutes after the reaction...
example 2
[0029] A reaction was carried out in the same manner as in Example 1 except for supplying methane, carbon dioxide, and steam at a molar ratio of CO2 / CH4=1.0 and H2O / CO2=0.1. The conversion rate of methane was measured in the initial stage of the reaction and two days after initiation of the reaction. The results are shown in Table 1.
example 3
[0030] A reaction was carried out in the same manner as in Example 1 except for supplying methane, carbon dioxide, and steam at a molar ratio of CO2 / CH4=1.0 and H2O / CO2=0.3. The conversion rate of methane was measured in the initial stage of the reaction and two days after initiation of the reaction. The results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com