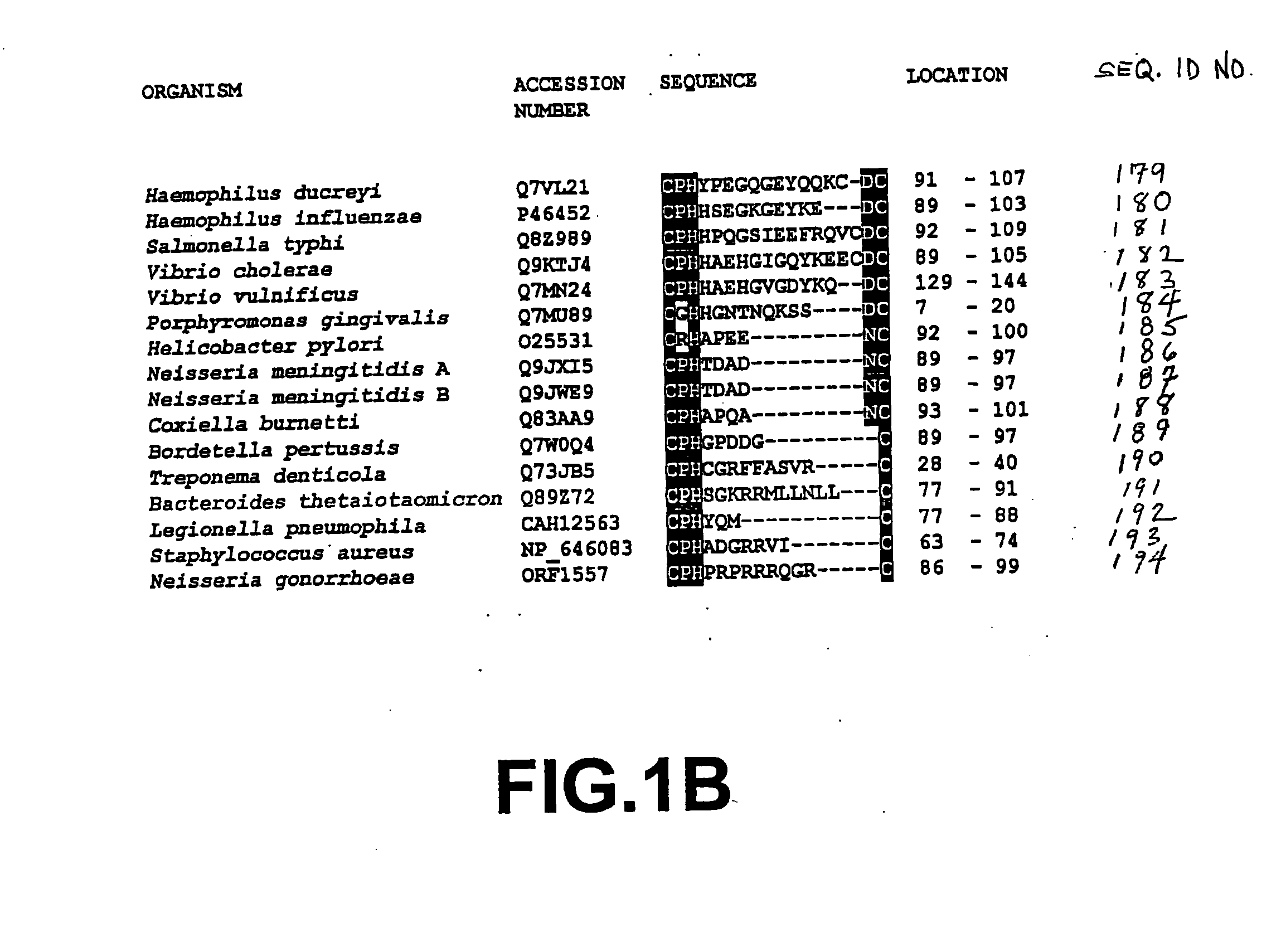
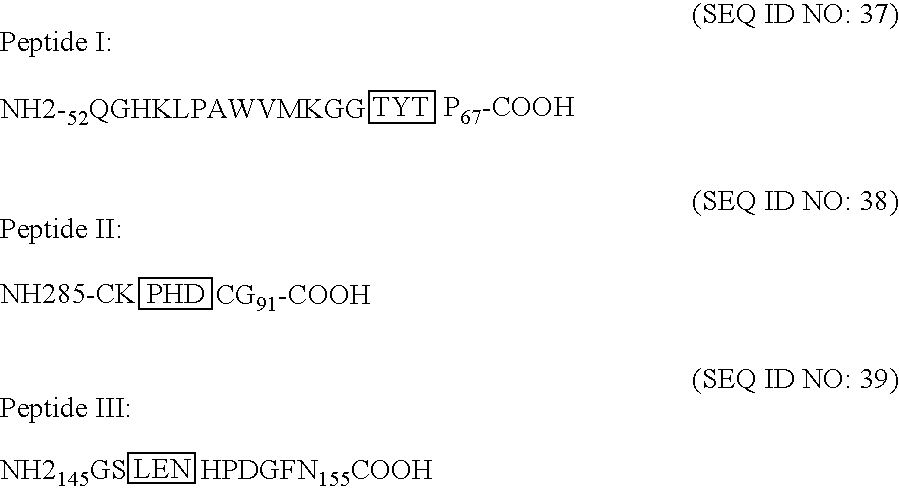Diagnosis and treatment of Alzheimer disease
a technology for alzheimer's and diagnosis, applied in the direction of bacteria, antibacterial agents, peptide/protein ingredients, etc., can solve the problems of lack of sensitivity required for detecting early stage or low level infections, syphilis does not account for the binding of some antibodies to complexes formed, and is not generally used as a diagnosti
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
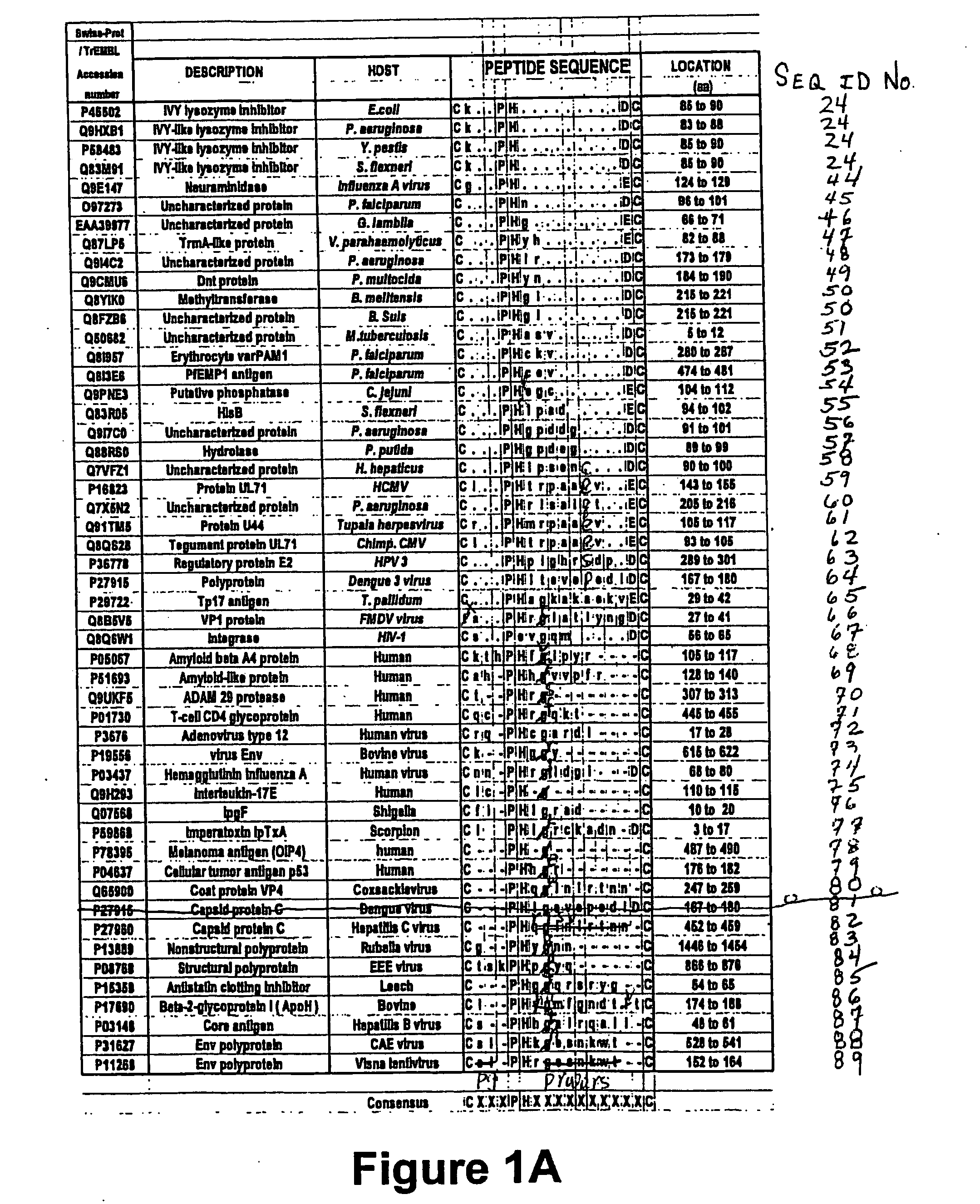
Chicken Lysozyme Copurifies with Recombinant Forms of the Treponema Pallidum 17 kDa Antigen
[0232] In the context of purifying E. coli-derived, recombinant T. pallidum Tp17 antigen (GST-Tp17 or Tp17-HIS), a protein reproducibly co-purified with Tp17. This “contaminant” protein, which had a molecular weight of 14 KDa and did not react with an anti-Tp17 polyclonal serum, was identified as chicken lysozyme as described below.
[0233] A protein fraction containing both entities (Tp17 and the “contaminant”) was separated by PAGE-SDS, excised from the gel and subjected to N-terminal amino acid sequencing. The experiment yielded short N-terminal peptide sequences (6-7 amino acids) that matched perfectly with the expected sequence of the T. pallidum Tp17 antigen or chicken lysozyme, which was included in the cell paste resuspension buffer to facilitate bacterial cell lysis. These observations suggested that Tp17 interacts physically with chicken lysozyme. Experimental protocols corresponding...
example 2
Far Western Detection of Tp17 Binding to Chicken and Human Lysozyme
[0245] The observation that the T. pallidum 17 kDa (Tp17) protein antigen copurifies with chicken lysozyme strongly suggested a direct physical interaction between both proteins. In order to test this hypothesis, a Far-Western blot lysozyme-protein interaction assay was performed. The assay is summarized in FIG. 5 and comprises generally the following three steps: (1) immobilization of purified lysozyme onto a membrane; (2) probing of the membrane with a ligand likely to bind directly to the lysozyme; and (3) immunodetection of the bound lysozyme-ligand. A detailed experimental procedure is presented below.
[0246] Far Western Blotting Detection of Lysozyme-Tp17 Protein Interactions
[0247] Purified chicken egg white lysozyme and human breast milk lysozyme were purchased from Sigma-Aldrich (Madrid, Spain). Two series of aliquot fractions containing 1 μg, 5 μg, and 10 μg of purified lysozyme and a protein molecular wei...
example 3
The T. Pallidum Tp17 Protein Antigen Inhibits the Antibacterial Activity of Chicken and Human Lysozyme
[0249] Lysozymes are well characterized antibacterial agents found on mucosal surfaces and in biological fluids. Due to their potent acetyl-muramidase enzymatic activity, lysozymes are capable of hydrolyzing cell wall peptidoglycan, thereby killing many pathogenic bacteria. The strong binding between Tp17 and human lysozyme suggested that this binding may alter the antibacterial activity of lysozyme. This hypothesis is consistent with the observation that (i) T. pallidum is a mucosal pathogen and (ii) it is in contact with human lysozyme in its ecological niche, throughout its infectious life cycle.
[0250] To test this hypothesis, the antibacterial activity of both human and chicken lysozyme, in the presence or absence of GST-Tp17, was assayed using an EnzCheck® lysozyme assay kit (Molecular Probes, Eugene, Oreg.). The assay comprises the use of Fluorescein-labeled Micrococcus lyso...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar ratio | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com