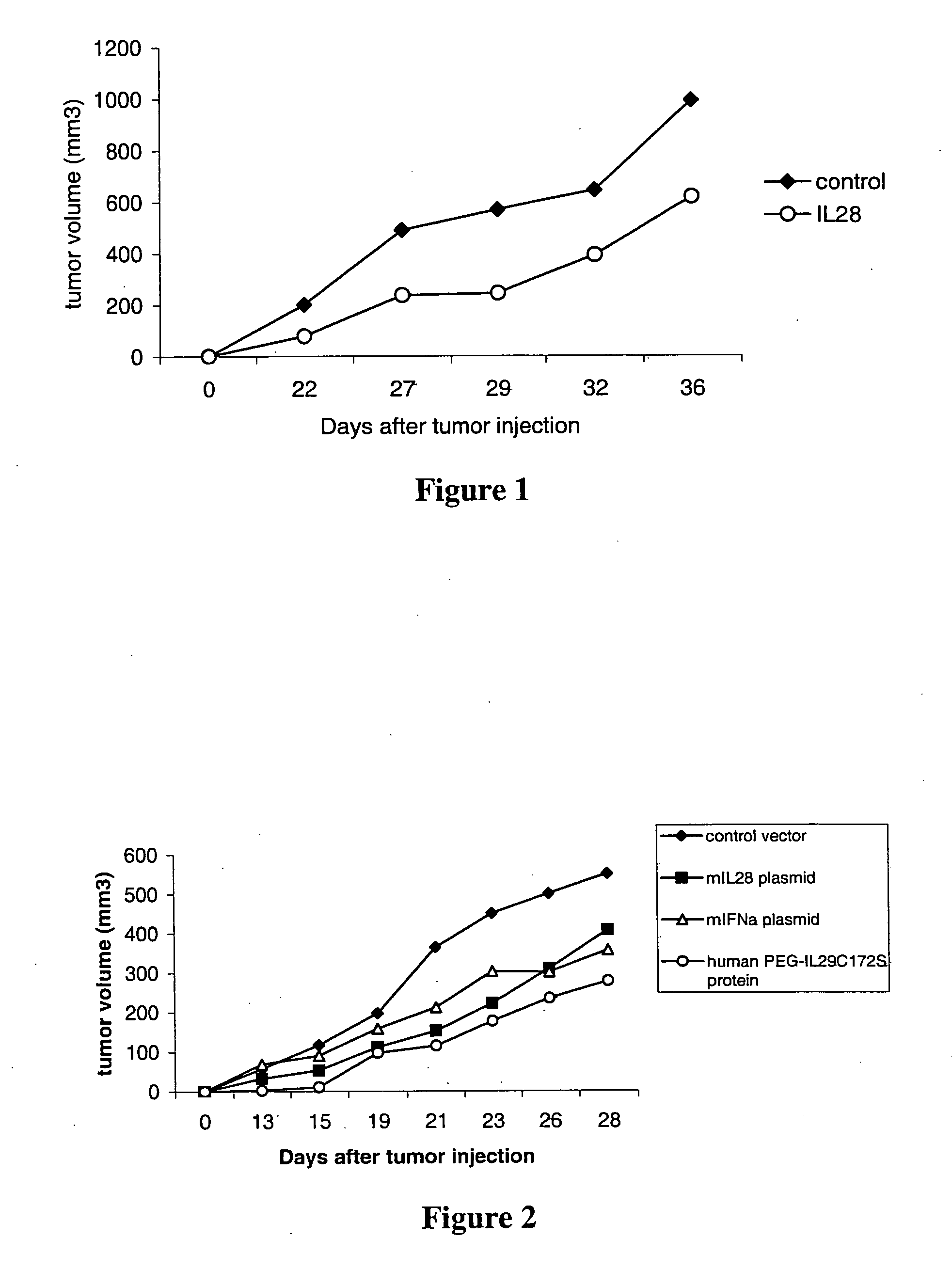
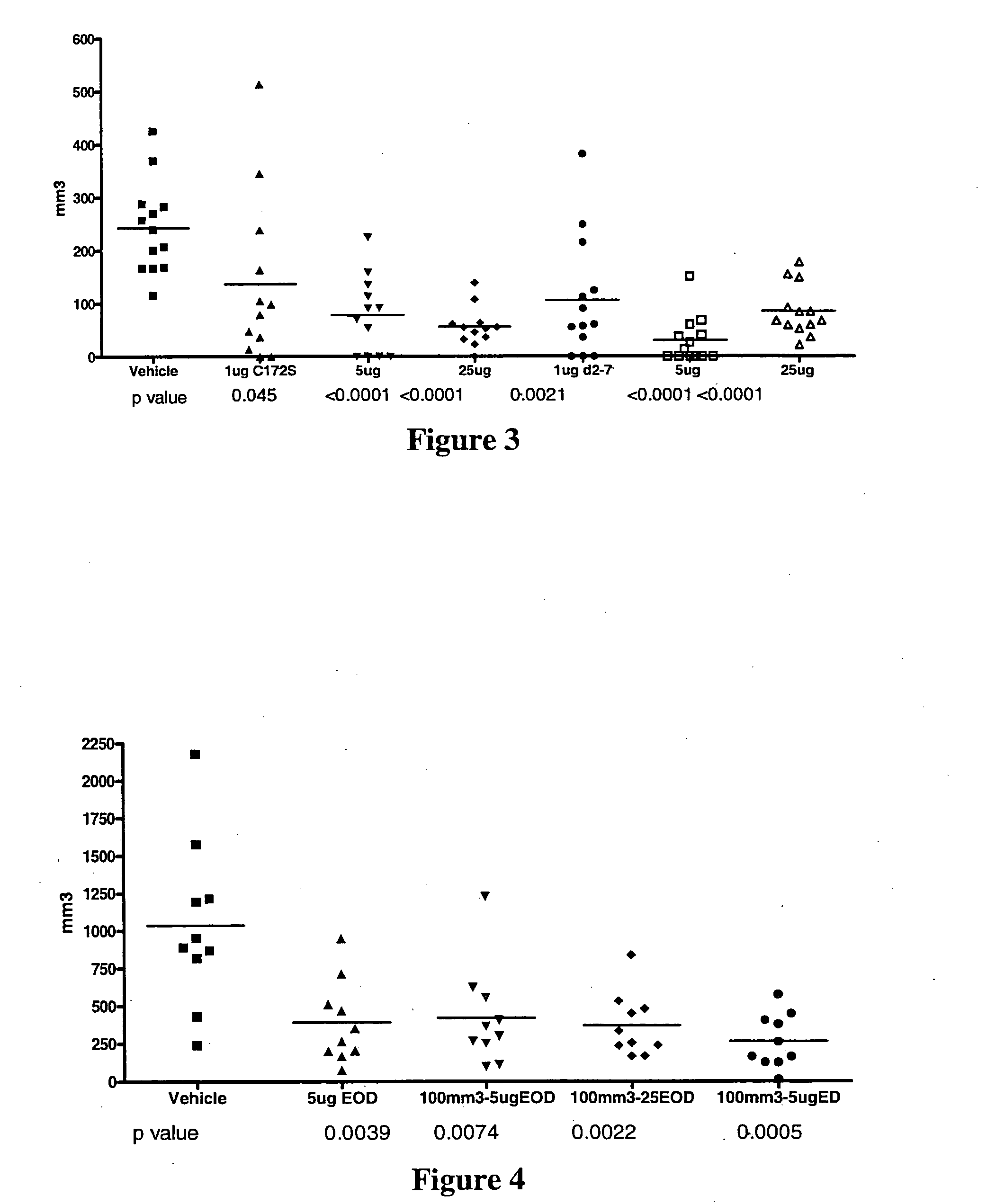
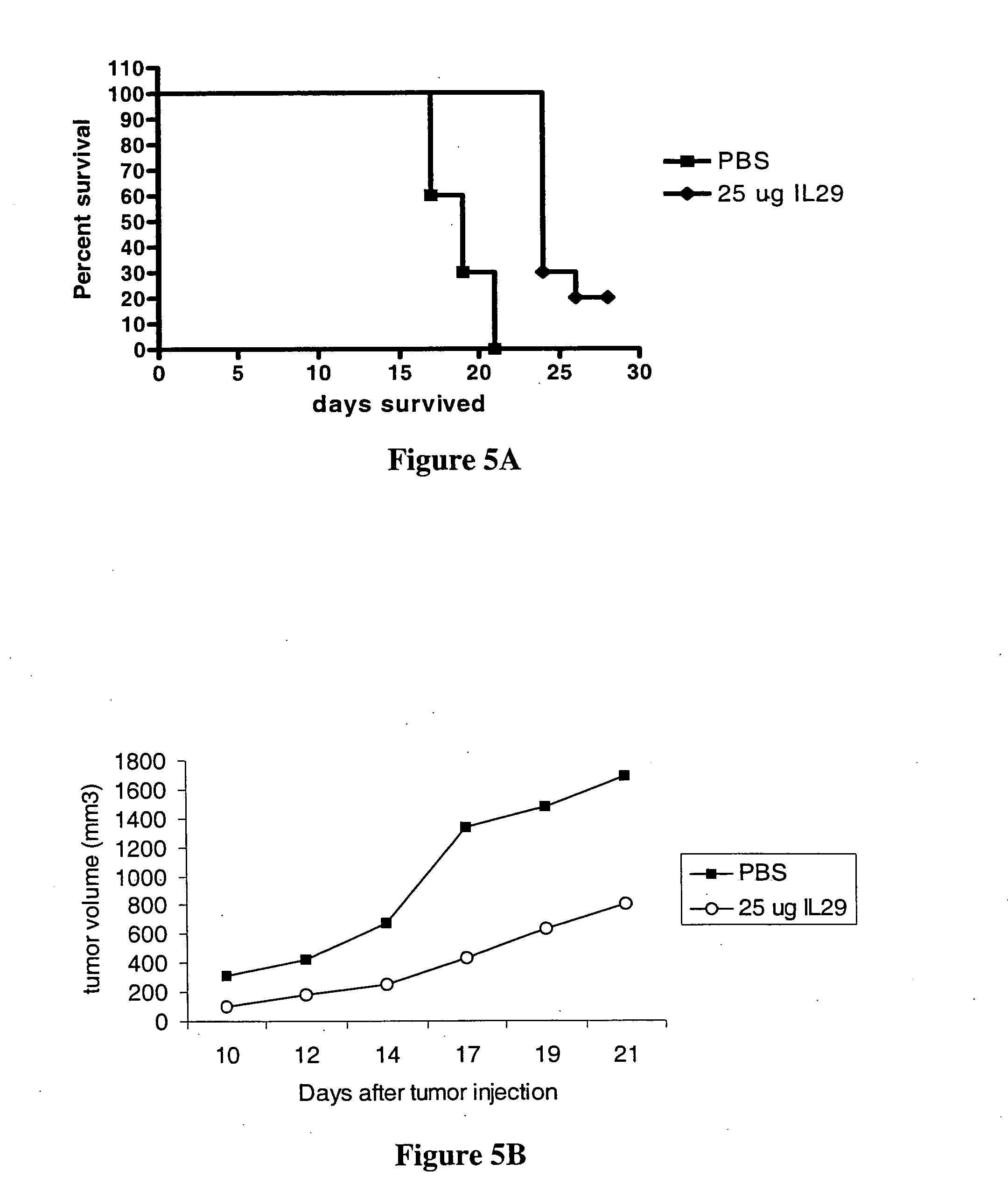
Use of truncated cysteine IL28 and IL29 mutants to treat cancers and autoimmune disorders
a technology of truncated cysteine and il28, which is applied in the field of use of truncated cysteine il28 and il29 mutants to treat cancers and autoimmune disorders, can solve the problems of loss of tolerance, scarring damage to nerve fibers, and disruption of neurotransmission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Mammalian Expression Plasmids
[0235] An expression plasmid containing zcyto20 and zcyto21 was constructed via homologous recombination. Fragments of zcyto20 and zcyto21 cDNA were generated using PCR amplification. The primers for PCR were as follows:
[0236] zcyto20 / pZMP21: zc40923, and zc43152 SEQ ID NOS: 42 and 43, respectively; and zcyto21 / pZMP21: zc40922, and zc43153 SEQ ID NOS:72 and 73, respectively.
[0237] The PCR reaction mixture was run on a 1% agarose gel and a band corresponding to the size of the insert was gel-extracted using a QIAquick™ Gel Extraction Kit (Qiagen, Valencia, Calif.).
[0238] The plasmid pZMP21, which was cut with BglII, was used for recombination with the PCR insert fragment. Plasmid pZMP21 is a mammalian expression vector containing an expression cassette having the MPSV promoter, and multiple restriction sites for insertion of coding sequences; an E. coli origin of replication; a mammalian selectable marker expression unit comprising an SV40 promoter, e...
example 2
Expression of Mammalian Constructs in CHO Cells
[0242] 200 μg of a zcyto20 / pZMP21 and zcyto21 / pZMP21 construct were digested with 200 units of Pvu I at 37° C. for three hours and then were precipitated with IPA and spun down in a 1.5 mL microfuge tube. The supernatant was decanted off the pellet, and the pellet was washed with 1 mL of 70% ethanol and allowed to incubate for 5 minutes at room temperature. The tube was spun in a microfuge for 10 minutes at 14,000 RPM and the supernatant was aspirated off the pellet. The pellet was then resuspended in 750 μl of PF-CHO media in a sterile environment, and allowed to incubate at 60° C. for 30 minutes. CHO cells were spun down and resuspended using the DNA-media solution. The DNA / cell mixture was placed in a 0.4 cm gap cuvette and electroporated using the following parameters: 950 μF, high capacitance, and 300 V. The contents of the cuvette were then removed and diluted to 25 mLs with PF-CHO media and placed in a 125 mL shake flask. The fl...
example 3
Purification and Analysis of zcyto20-CHO Protein
[0243] Purification of Zcyto20-CHO Protein
[0244] Recombinant zcyto20 (IL-28A) protein was produced from a pool of DXB11-CHO cell lines. Cultures were harvested, and the media were sterile filtered using a 0.2 μm filter.
[0245] The purification of zcyto20-CHO protein was achieved by the sequential use of a Poros HS 50 column (Applied Biosystems, Framingham, Mass.), a Monolithic WCX column (Isco, Inc., Lincoln, Nebr.), a ToyoPearl Butyl 650S column (TosoH, Montgomeryville, Pa.), and a Superdex 75 column (Amersham Biosciences, Piscataway, N.J.). Culture media from DXB111-CHO were adjusted to pH 6.0 before loading onto a Poros 50 HS column. The column was washed with 50 mM MES (2-Morpholinoethanesulfonic acid), 100 mM NaCl, pH 6 and the bound protein was eluted with a 10 column volumes (CV) linear gradient to 60% of 50 mM MES, 2 M NaCl, pH 6. The eluting fractions were collected and the presence of zcyto20 protein was confirmed by SDS-PA...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com