Pharmacological chaperones for treating obesity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Generation of Cell Lines Expressing MC4R Folding Mutants
[0175] In order to determine whether some MC4R mutants result in conformational defects of MC4R, MC4R nucleic acids containing the various mutants are transfected into HEK-293T and COS-7 cells and their cell surface expression and activity evaluated. Those cell lines in which reduced or absent cell surface expression is observed are evaluated further to determine the intracellular presence and / or location of the MC4R polypeptide.
Methods
[0176] Generation of MC4R Mutants. A cDNA encoding a wild-type MC4R (e.g., SEQ ID NO: 1) is modified using known techniques in the art (e.g., PCR, site-directed mutagenesis) to generate mutant MC4R cDNAs containing alterations in the nucleotides which result in, e.g., one of the following MC4R mutant polypeptides: P78L, R165Q, R165W, 1125K, C271Y, T11A, A175T, I316L, I316S, I317T, N97D, G98R, N62S, C271R, S58C, N62S, N97D, Y157S, I102S, L106P, L250Q, Y287X, P299H, S58C, CTCT at codon 211, and / ...
example 2
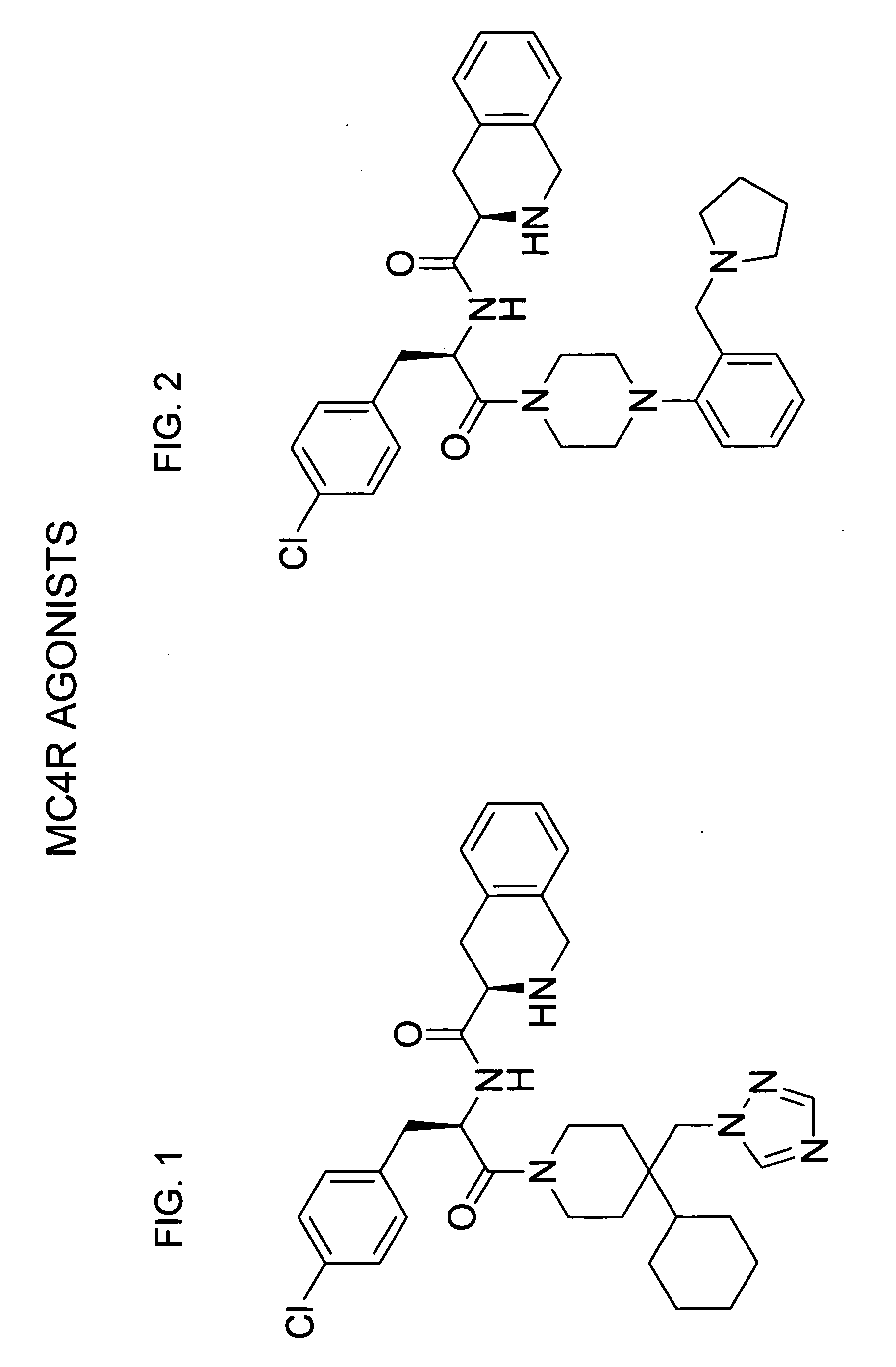
Structures of Agonists and Antagonists of MC4R
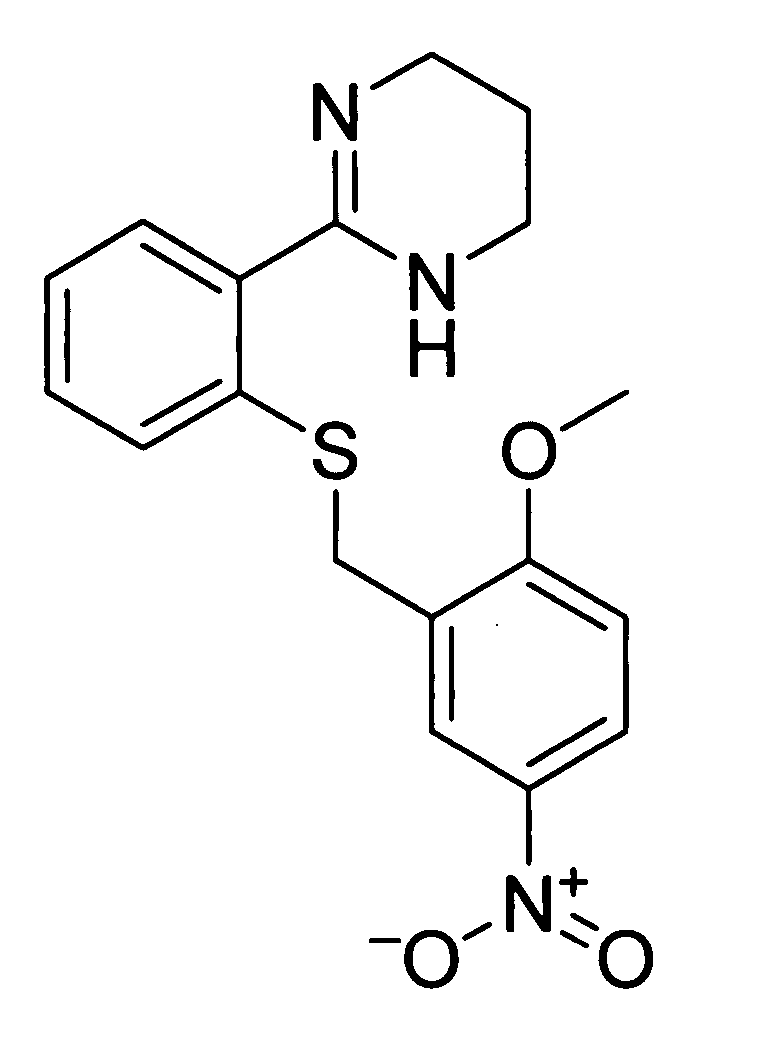
[0184] Potential agonists and antagonists of MC4R were selected based on the review of published patent and literature references (in particular, Bednarek and Fong, Exp. Opn. Therapeutic Patents 2004; 14(3): 327-326 and WO 02 / 062766). Criteria used in selecting the compounds described herein included published IC50 data, in vivo animal data, and bioavailability data (e.g., pharmacokinetics), where available.
[0185] a) Synthesis of the Agonist of FIG. 1 (compound 1)
[0186] Selection of this compound, known as THIQ, was based on the following data:
MC4R ActivityIC50EC50EmaxName(nM)(nM)(%)PKReference(s)THIQ / 1.22.597% F14Van der Ploeg et al (2002)compound 1Vd3.6 L / kgPNAS 99: 11381.Cl84 mLmin / kgSebhat et al (2002) J Medt1 / 20.6 hChem 45: 4589.
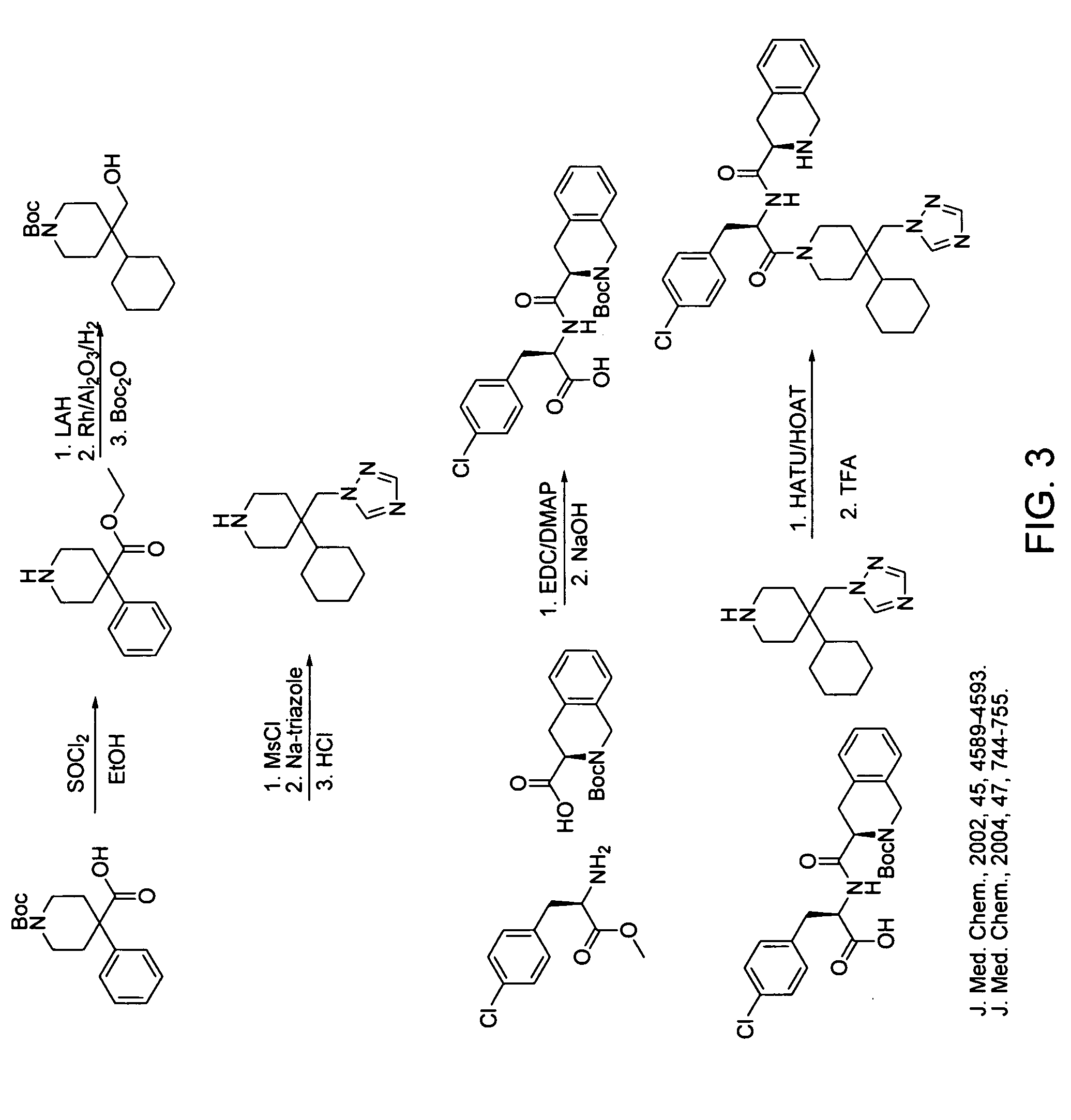
[0187] Synthesis of this molecule (11-steps) was performed based on the scheme depicted in FIG. 3.
[0188] b) Synthesis of the Agonist of FIG. 2 (compound 2)
[0189] Selection of this known compound, refe...
example 3
Rescue of Misfolded MC4R Using Low Temperature or Chemical Chaperones
[0198] Since both low temperatures (thermal rescue) and general chemical chaperones such as DMSO are known to restore folding of mutant proteins, cell surface expression of wild-type and various MC4R folding mutants was evaluated in cells harboring WT and mutant MC4R, and in those cells cultured at 30° C. or with 1% DMSO.
Methods
[0199] Cells and Transfections. HEK 293 cells were transiently transfected with wild-type (WT) or the following hMC4R mutants double tagged with 3HA and Venus (Enhanced Yellow Green Fluorescent Protein; EYFP): S58C; N62S; R165W; R165Q, and P299H.
[0200] Low Temperature Assay. WT and transfected cells were incubated at 30° C. or 37° C. for 12 hours prior to evaluation of MC4R cell surface expression. The gain was determined using the following: Gain=[(% of surface expression at 30° C.−% of surface expression at 37° C.) / % of surface expression at 37° C.]*100.
[0201] Chemical Chaperone Assay...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com