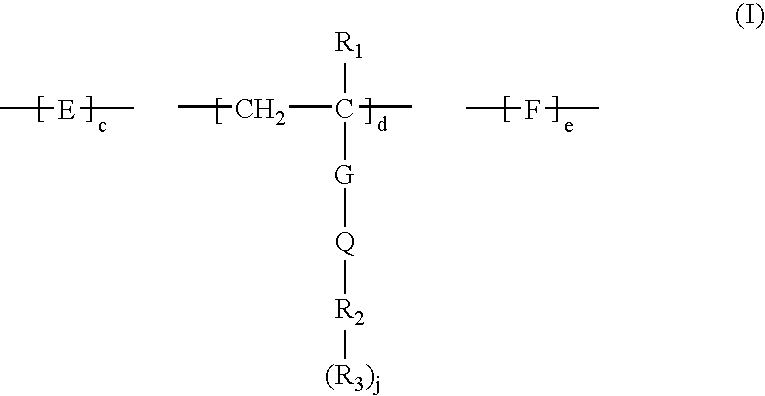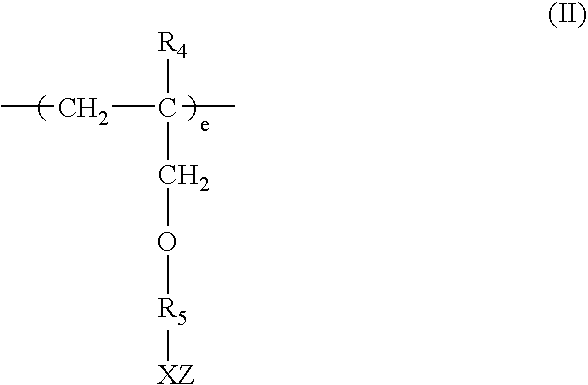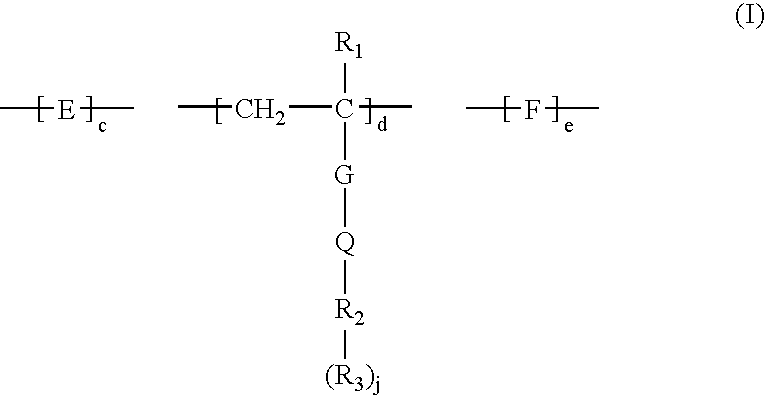Method for removing calcium from crude oil
a crude oil and calcium technology, applied in the petroleum industry, hydrocarbon oil refining, etc., can solve the problems of residual product contamination, heavy damage to refinery tower trays and other equipment, and solids other than salts are equally harmful,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0043] In order to assess the efficacy of various candidate materials in inhibiting calcium citrate crystal formation, a solution (solution A) of 1,000 ppm (as solids) calcium chloride, and 1,000 ppm (as solids) citric acid was prepared. NaOH was added to bring the pH up to 7.1. Treated and untreated solutions were heated at 100° C. for 1-1.5 hours. Results are shown in Table 1.
TABLE 1TreatmentObservations1100 ml solution A: untreatedA lot of fine crystals precipitated on bottom(assumed 100%). The water is clear.2100 ml solution A + sulfuric acid dilutionAbout 25% (compare to the untreated)to have pH 5.1crystallize growing.The water is clear.3100 ml solution A + sulfuric acid dilutionAbout 40% (compare to the untreated)to have pH 6.1crystallize growing.The water is clear.4100 ml solution A + 50 ppm activeA lot of fine and floc precipitate.HEDP (DeQuest 2010)The water is cloudy.5100 ml solution A + 50 ppm active NTAA few (water is clear.6100 ml solution A + 50 ppm ComparativeA lot ...
example 2
[0044] Additional tests utilizing the procedure of Example 1 were conducted. Results are reported in Table 2.
TABLE 2TreatmentObservations2.1100 ml solution A: untreatedA lot of fine crystals precipitated onbottom (assumed 100%). The water isclear.2.2100 ml solution A + 10 ppm active NTAA lot of fine crystals precipitated onbottom (about 100%). The water is clear.2.3100 ml solution A + 20 ppm active NTAA lot of fine crystals precipitated onbottom (about 60%). The water is clear.2.4100 ml solution A + 30 ppm active NTALesser fine crystals precipitated onbottom (about 30%). The water is clear.2.5100 ml solution A + 40 ppm active NTAAbout 5% crystals on bottom.The water is clear.2.6100 ml solution A + 50 ppm active NTAVery few crystals on bottom.The water is clear.
example 3
[0045] Further tests utilizing the procedure of Example 1 were undertaken. Results are shown in Table 3.
TABLE 3TreatmentObservations3.1100 ml solution A: untreatedA lot of fine crystals precipitated onbottom (assumed 100%), the water isclear water. 0.0595 g crystals3.2100 ml solution A + 35 ppm active NTAAbout 5-10% crystals on bottom.The water is clear.3.3100 ml solution A + 35 ppm active EDTA-About 5-10% crystals on bottom.free acidThe water is clear.3.4100 ml solution A + 70 ppm Product AClean and clear water. No crystals.3.5100 ml solution A + 70 ppm Product BClean and clear water. No crystals.3.6100 ml solution A + 70 ppm Product PBTCAbout 5-10% crystals on bottom.The water is clear.3.7100 ml solution A + 70 ppm ProductNo crystals observed, but the water isDeQuest 2060cloudy.3.10100 ml solution A + 30 ppm Product AClean and clear water. No crystals.3.11100 ml solution A + 50 ppm Product AClean and clear water. No crystals.3.12100 ml solution A + 70 ppm Product AClean and clea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com