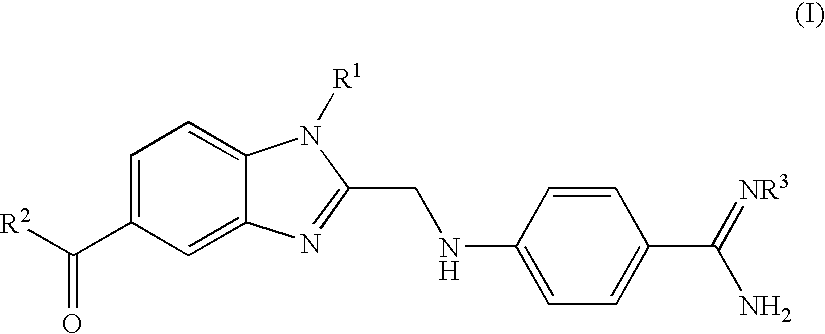
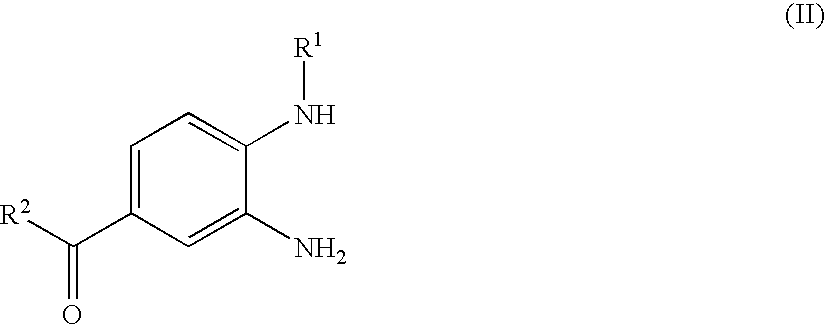
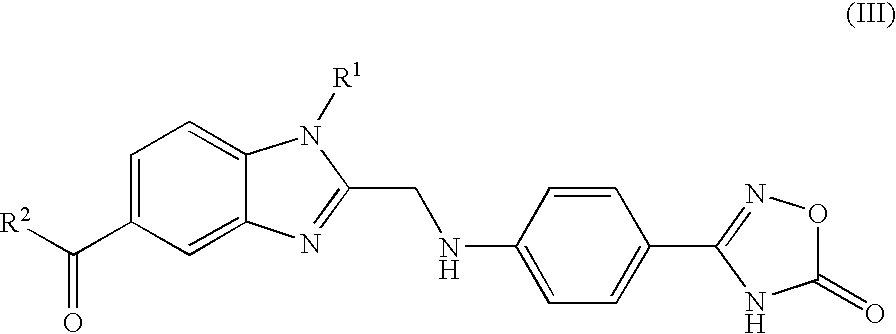
Process for the Preparation of 4-(Benzimidazolylmethylamino)-Benzamides and the Salts Thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 2-[4-(1,2,4-oxadiazol-5-on-3-yl)-aniline (1)
Variant 1:
[0105] (1A)
[0106] In the reaction vessel are placed 118.6 g (1 mol) 4-aminobenzonitrile and 68.9 g (0.65 mol) sodium carbonate in 500 ml of ethanol and 100 ml of water and the mixture is heated to 60° C. 76.4 g (1.1 mol) hydroxylamine-hydrochloride, dissolved in 100 ml of water, are slowly added dropwise to this suspension.
[0107] The mixture is subsequently stirred overnight at 60° C. During cooling to 0-5° C. the substance is precipitated, filtered off and washed several times with a total of 150 ml cold water and 100 ml cold ethanol. Finally the mixture is washed with 50 ml MtBE and 178.4 g moist product are obtained. This is dried at 35° C. in vacuo.
[0108] Yield: 135.4 g light beige substance (89.5% of theory), melting point: from 169.5° C. (decomp.); purity: >98% HPLC peak area
[0109] (1B)
[0110] 25.02 g (0.46 mol) sodium methoxide are added batchwise to a suspension of 60.5 g (1A) (0.4 mol) in 400 ml of ...
example 2
Preparation of 2-[4-(1,2,4-oxadiazol-5-on-3-yl)-phenylamino]-acetic acid (2)
Variant 1:
[0119] (2A)
[0120] At ambient temperature 83.5 g (0.5 mol) ethyl bromoacetate are metered into a suspension of 70.86 g (0.4 mol) (1B) and 26.5 g (0.25 mol) sodium carbonate in 600 ml of water / isopropanol and stirred overnight. The reaction mixture is reddish-brown to orange. The suspension cooled to 0° C. is suction filtered, washed in several batches with 300 ml of water and 150 ml of ethanol (106 g moist light-brown substance) and dried at 35° C. in vacuo.
[0121] Yield: 92.44 g brownish substance (87.7% of theory) melting point: from 186.1° C. (decomp.); purity: >98% HPLC peak area
[0122] (2B)
[0123] The ester (2A) (86.9 g; 0.33 mol) thus obtained is suspended in 400 ml of water and at RT 120 g of 45% NaOH are slowly added dropwise. The suspension goes into solution and is reddish (pH 12.5). It is heated to 60° C. and saponified for 1 h. The solution obtained is combined batchwise with HCl (37...
example 3
Preparation of 3-amino-4-methylaminobenzoic acid-N-(2-pyridyl)-N-(2-ethoxycarbonylethyl)-amide (AMBPA) (3)
[0131]
Variant A: Pd / C 5%
[0132] 150 g (0.4 mol) 4-methylamino-3-nitrobenzoic acid-N-(2-pyridyl)-N-(2-ethoxycarbonylethyl)-amide, 12 g 5% palladium on charcoal catalyst and 627 ml of ethyl acetate are placed in a hydrogenating autoclave. The mixture is hydrogenated under a hydrogen atmosphere of 3-4 bar at 35-55° C. until the hydrogen uptake is constant (1-2 h). After cooling to 20° C. the hydrogenating solution is filtered off from the catalyst and evaporated down in vacuo using the rotary evaporator. The residue is taken up in 650 ml isopropanol, distilled down to half the original volume and cooled to 5-10° C. After 4 h the resulting suspension is filtered, and the precipitate thus isolated is washed batchwise with a total of 100 ml isopropanol. The solid obtained is dried in the vacuum dryer at 50° C.
[0133] Yield: 114.2 g (corr. 83% of theory)
Variant B: Pd / C 10%
[0134] 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com