Extended release of neuregulin for improved cardiac function
a neuregulin and cardiac function technology, applied in the direction of drug compositions, peptide/protein ingredients, metabolic disorders, etc., can solve the problems of ace inhibitor mortality reduction of 3%, lack of robust cell-cell interactions, size and accumulation of contractile proteins, etc., to enhance the production and/or function of said nrg, improve the effect of nrg, and reduce adverse side effects of nrg
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
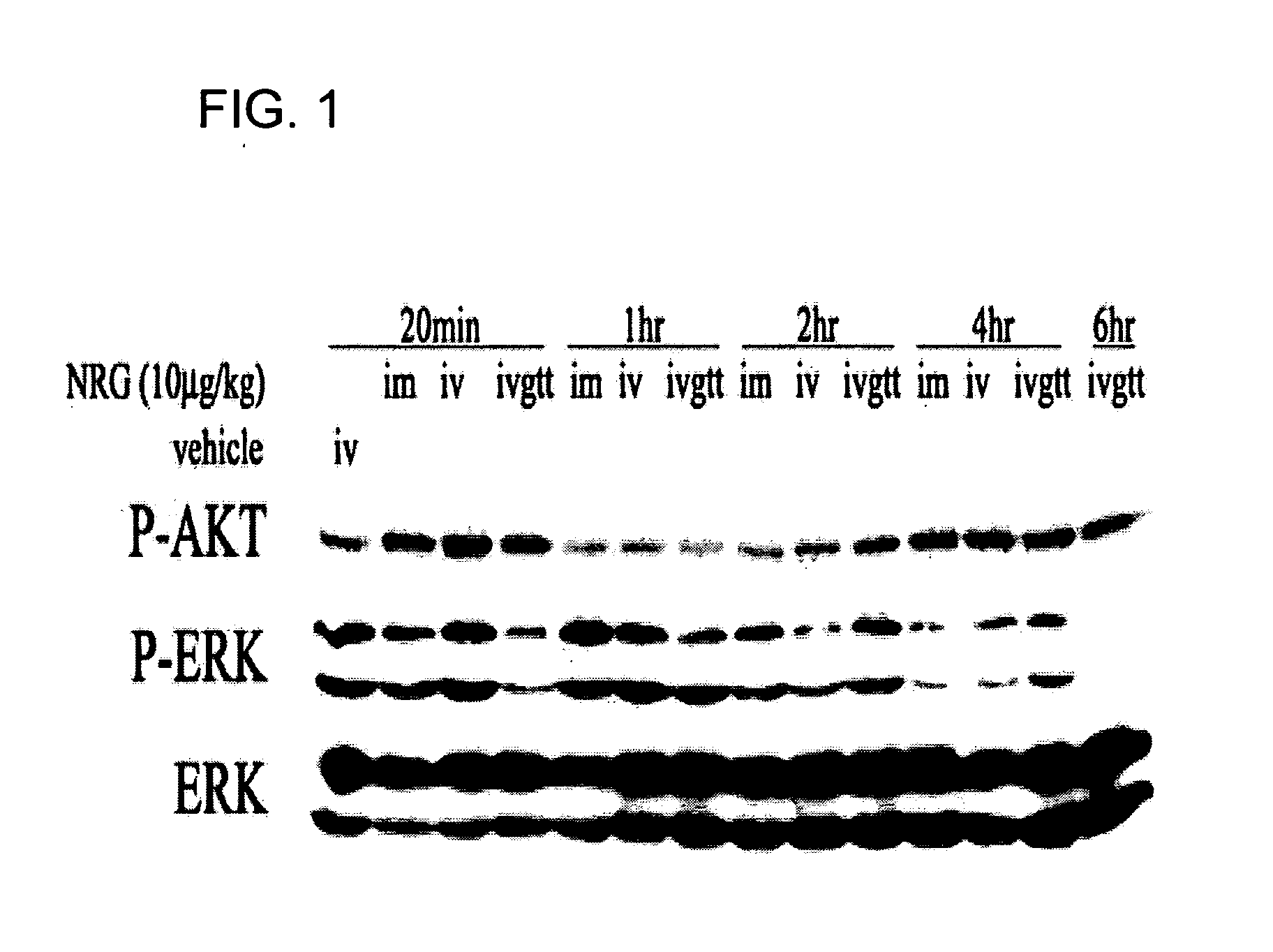
[0161] Phosphorylation of AKT and ERK in the Left Ventricle of Normal Rats after NRG is Infused by Different Methods.
[0162] To compare the effect of NRG with various treating methods on the signal transduction inside the cardiac myocytes in the left ventricle, we infused NRG by intravenous (hereinafter referred to as “IV”), intramuscular (hereinafter referred to as “IM”) and IV glucose tolerance test (hereinafter referred to as “IVGTT”).
[0163] Wistar male rats (Shanghai Animal Center of Chinese Academy of Science), which weighed 180±20 grams, were numbered, weighed, and divided into groups. Each group contained three rats. One group received IV injection of 4 ml / kg (volume / body weight) of vehicle (10 mM Na2HPO4-NaH2PO4, 150 mM NaCl, 0.2% human serum albumin (HSA), 5% mannitol, pH 6.0) as a control. Four other groups of rats received IM injection of 4 ml / kg (volume / body weight) of NRG (37.3 U / ml recombinant human NRG fragment (from the 177th to 237th amino acid sequence of human NR...
example 2
[0166] The Function of Left Ventricle Coronary Artery Ligated Rat Heart after Neuregulin Treatment by Different Methods
[0167] As osmotic pump is a way to deliver NRG constantly (as IVGTT), we examined whether NRG infused by osmotic pump was as effective as conventional IV injection in restoring the function of myocardial infarct (MI) heart.
A. Rat Left Ventricle Coronary Artery Ligation and Echocardiography
[0168] Wistar male rats (Shanghai Animal Center of Chinese Academy of Science), which weighed 200±20 g, were anesthetized by intraperitoneally injecting 100 mg / kg (drug / body weight) of ketamine. The neck and chest were depilated and sanitized. An incision was made in the middle front neck to expose the tracheae. An 18 G catheter overneedle was inserted into the tracheae between the 3rd and 5th cartilage of tracheae. After the needle was drawn out, a plastic cannula was pushed into the trachea 1-2 cm and fixed to connect the Rodent Ventilator (SAR-830 / P ventilator—Inspiratory fl...
example 3
[0182] Heart Function of Myocardial Infarcted Rats after Neuregulin was Constantly Intravenously Infused by Syringe Pump (Zhejiang University Medical Instrument Co. LTD, WZS 50-F2)
[0183] In this example, syringe pump is used for extended release of neuregulin in human patients. Syringe pump can pump the solution continuously at certain speed into the bloodstream through a needle injected into the vein in rat tail. For syringe pump, it's easy to control the infusion time and speed. Neuregulin was intravenously infused by syringe pump at different speed for different time per day into MI rats to better time period and speed for treatment.
[0184] Grouped MI rats was treated by intravenous injection of 4 ml / kg (volume / body weight) vehicle everyday for 10 days (group A); or intravenous injection of 10 μg / kg neuregulin (2.5 μg / ml) everyday for 10 days (group B); or intravenous syringe pump infusion of neuregulin (0.625 μg / ml) at 1.25 μg / kg / h with 4 hours per day for 10 days (group C); or...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface area | aaaaa | aaaaa |
| surface area | aaaaa | aaaaa |
| surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com