Method for nitriding metal in salt bath and metal manufactured using the same
a technology of salt bath and nitrided metal, which is applied in the direction of manufacturing tools, heat treatment equipment, furnaces, etc., can solve the problems of high cost, waste water and gas processing cost, and the nitriding method using cyanide salt, so as to improve the tensile strength of steel and iron, the surface hardness of the iron nitrided by the present invention is also improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
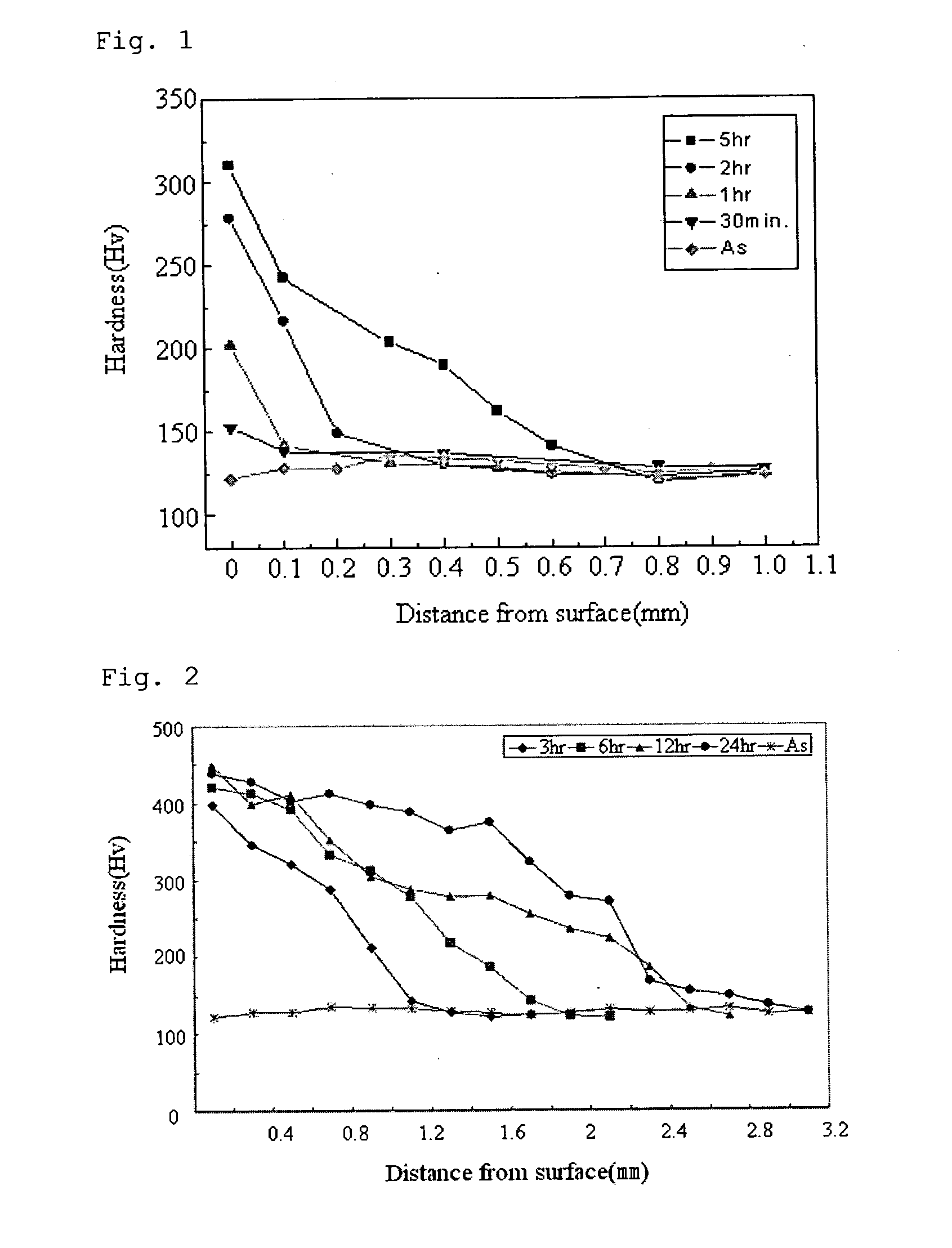
[0044]In accordance with the first embodiment of the present invention, steel is nitrided using the NaNO3 molten salt. The nitrided steel includes ultra-low carbon steel, low carbon steel, medium carbon steel, high carbon steel and alloy steel.
[0045]Each of the ultra-low carbon steel, low carbon steel, medium carbon steel, high carbon steel and alloy steel is submerged in the NaNO3 molten salt bath for 2 hours at a temperature of 500° C.
[0046]Table 2 shows changes in surface hardness and tensile strength of the samples nitrided in the molten salt bath, wherein the hardness was measured using a Vickers hardness tester under a load of 1 kgf.
[0047]In case of ultra-low carbon steel, the surface hardness increases by 119% and the tensile strength increases by 47%. In case of low carbon steel, the surface hardness increases by 47% and the tensile strength increases by 19%.
[0048]In case of medium carbon steel, the surface hardness increases by 32% and the tensile strength increases by 18%....
second embodiment
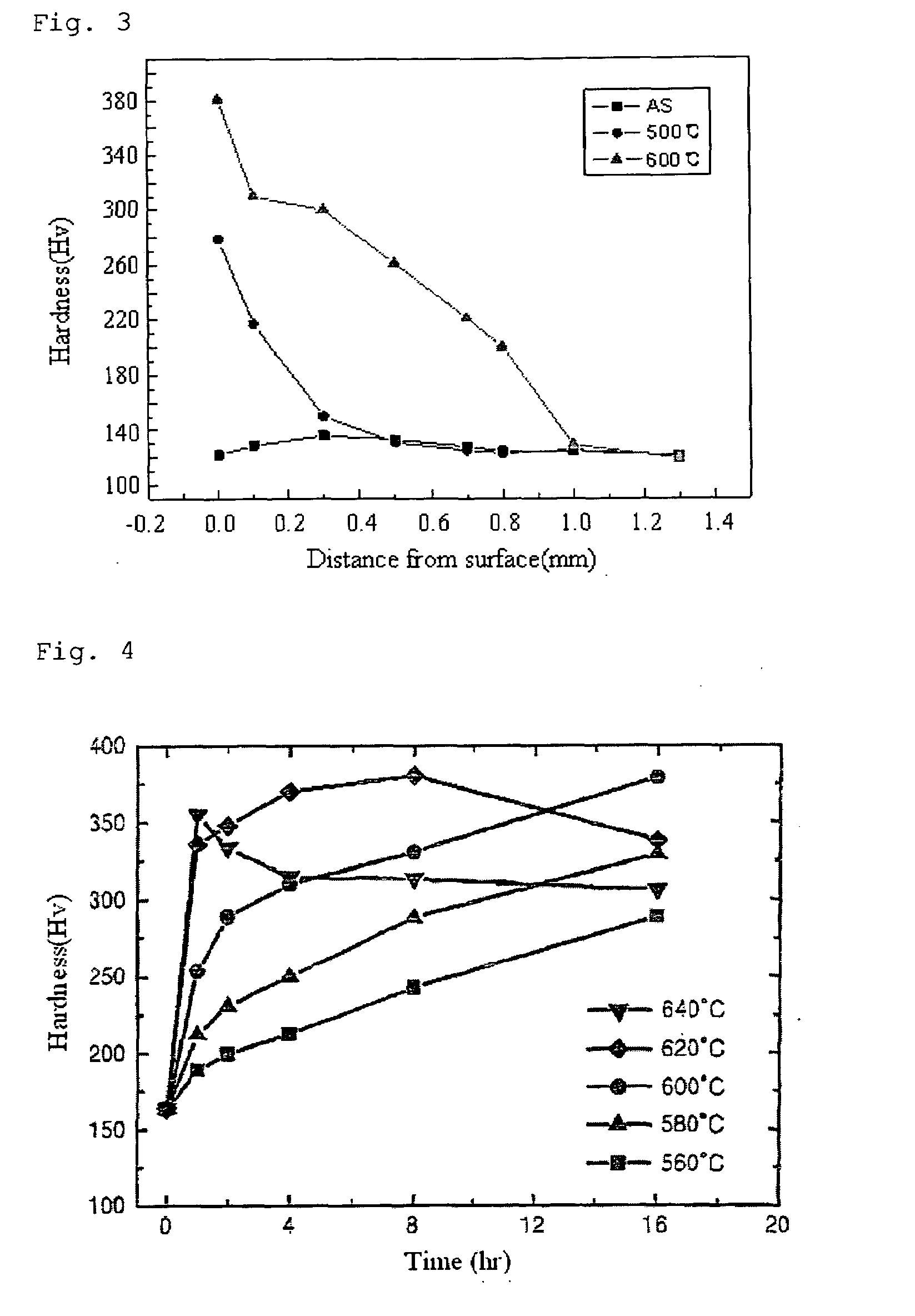
[0061]In accordance with the second embodiment of the present invention, steel is nitrided by using the NaNO2 molten salt.
[0062]Steels including ultra-low carbon steel, low carbon steel, medium carbon steel, high carbon steel and alloy steel are submerged in the salt bath at 450° C. for 2 hours.
[0063]Table 4 shows changes in surface hardness and tensile strength of the samples nitrided in the molten salt bath, wherein the surface hardness is measured using a Vickers hardness tester under a load of 1 kgf.
[0064]For ultra-low carbon steel, the surface hardness increases by 54% and the tensile strength increases by 21%. For low carbon steel, the surface hardness increases by 32% and the tensile strength increases by 15%.
[0065]For medium carbon steel, the surface hardness increases by 19% and the tensile strength increases by 13%. For high carbon steel, the surface hardness increases by 18% and the tensile strength increases by 12%.
[0066]For alloy steel, the surface hardness increases by...
third embodiment
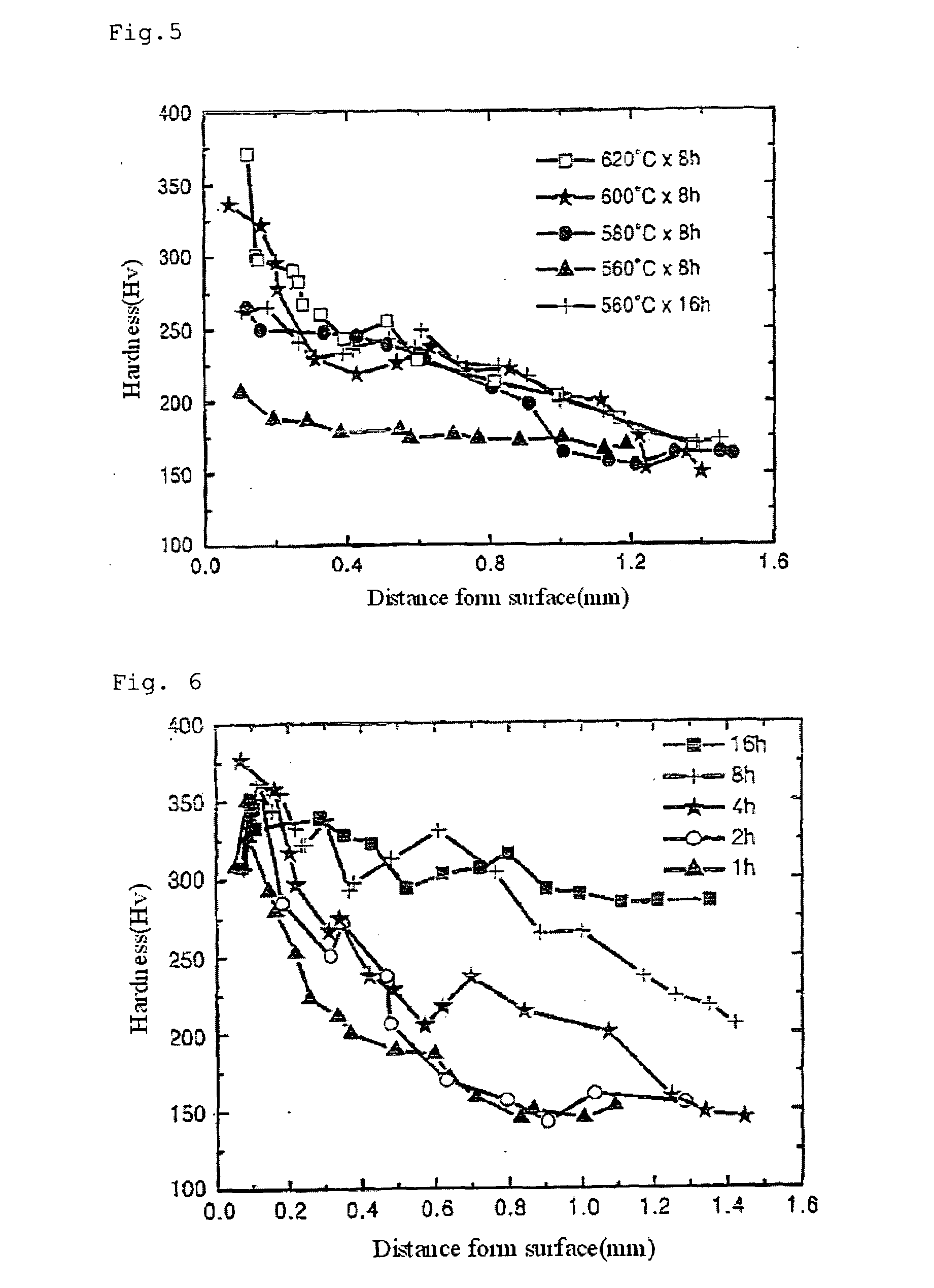
[0069]In accordance with the third embodiment of the present invention, steels are nitrided using the KNO2 molten salt.
[0070]The steels including ultra-low carbon steel, low carbon steel, high carbon steel and alloy steel are submerged in the molten salt bath at 480° C. for 2 hours.
[0071]Table 5 shows changes in hardness and tensile strength of the samples submerged in the molten salt bath, wherein the surface hardness is measured using a Vickers hardness tester under a load of 1 kgf.
[0072]For ultra-low carbon steel, the surface hardness increases by 45% and the tensile strength is increases by 15%. For low carbon steel, the surface hardness increases by 25% and the tensile strength increases by 11%.
[0073]For high carbon steel, the surface hardness increases by 17% and the tensile strength increases by 10%. For alloy steel, the surface hardness increases by 12% and the tensile strength increases by 11%.
[0074]That is, when the steels are nitrided using the molten salt bath nitriding ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com