Container for preserving blood products at cryogenic temperatures
a technology of cryogenic temperature and container, which is applied in the field of cryogenic temperature preservation of blood or blood components, can solve the problem of higher cos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
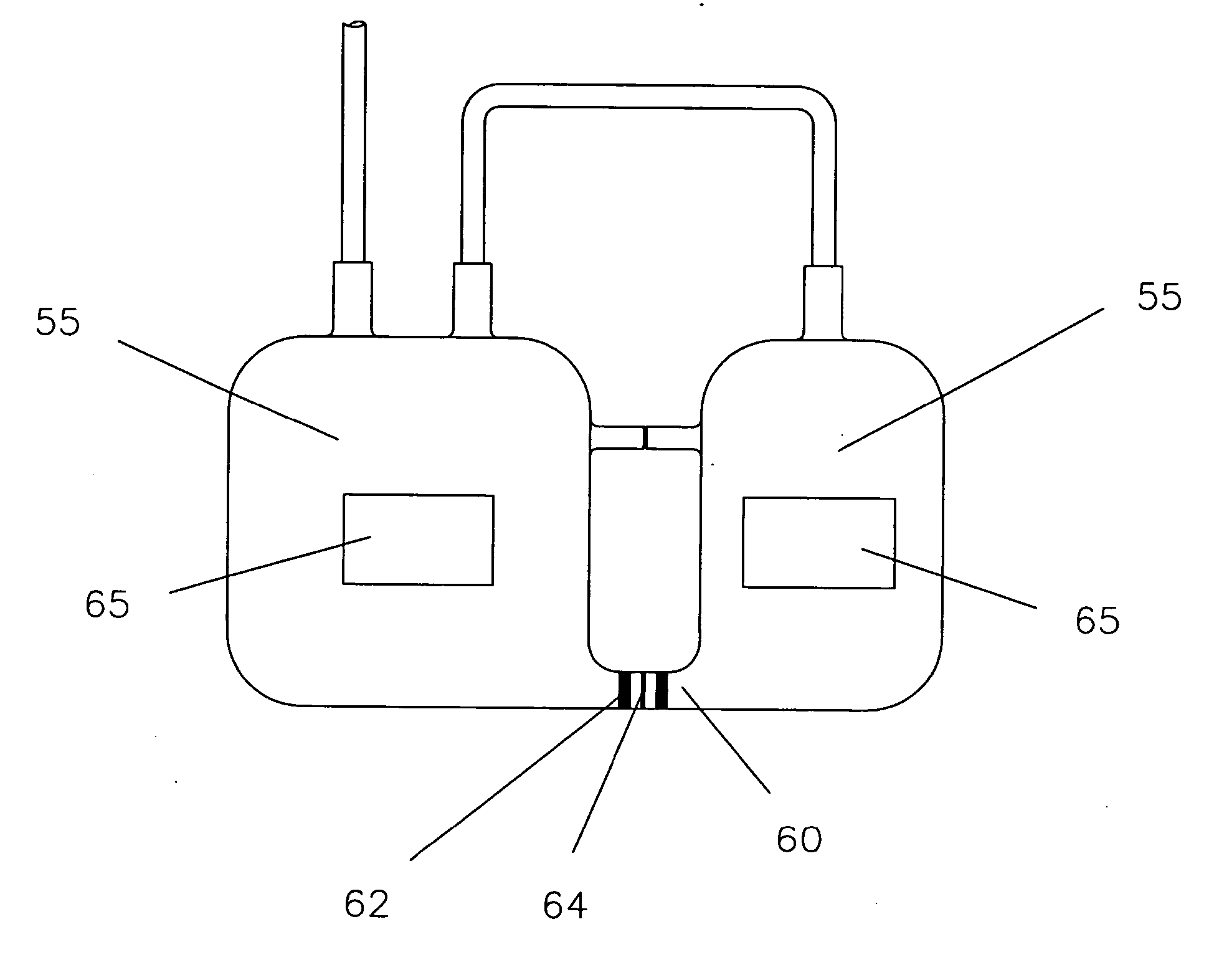
[0032] It is the essence of this invention to provide a durable and tough container that can reliably endure long-term storage at very low cryogenic temperature environments. Such containers are essential to preserve valuable and perishable contents such as rare blood or progenitor cells of neonatal. The present invention provides a metallic container characterized with high strength and high toughness to endure very low cryogenic temperature. It is known that cold temperatures embrittle materials causing it to fracture at much lower than normal impacts. By using tough metals that greatly minimize the effect of embrittlement, containers are capable of withstanding stresses or impacts at low temperatures.
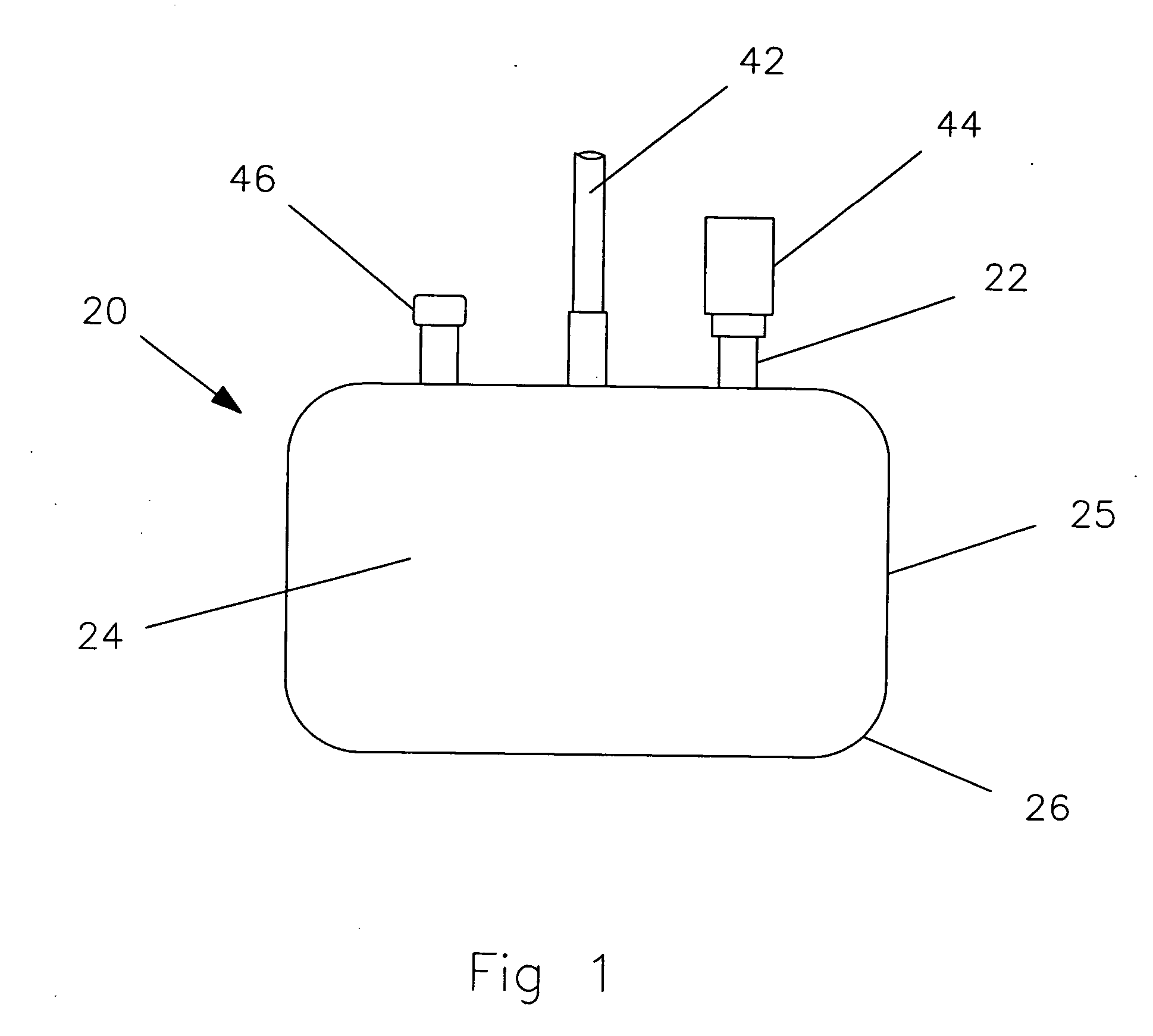
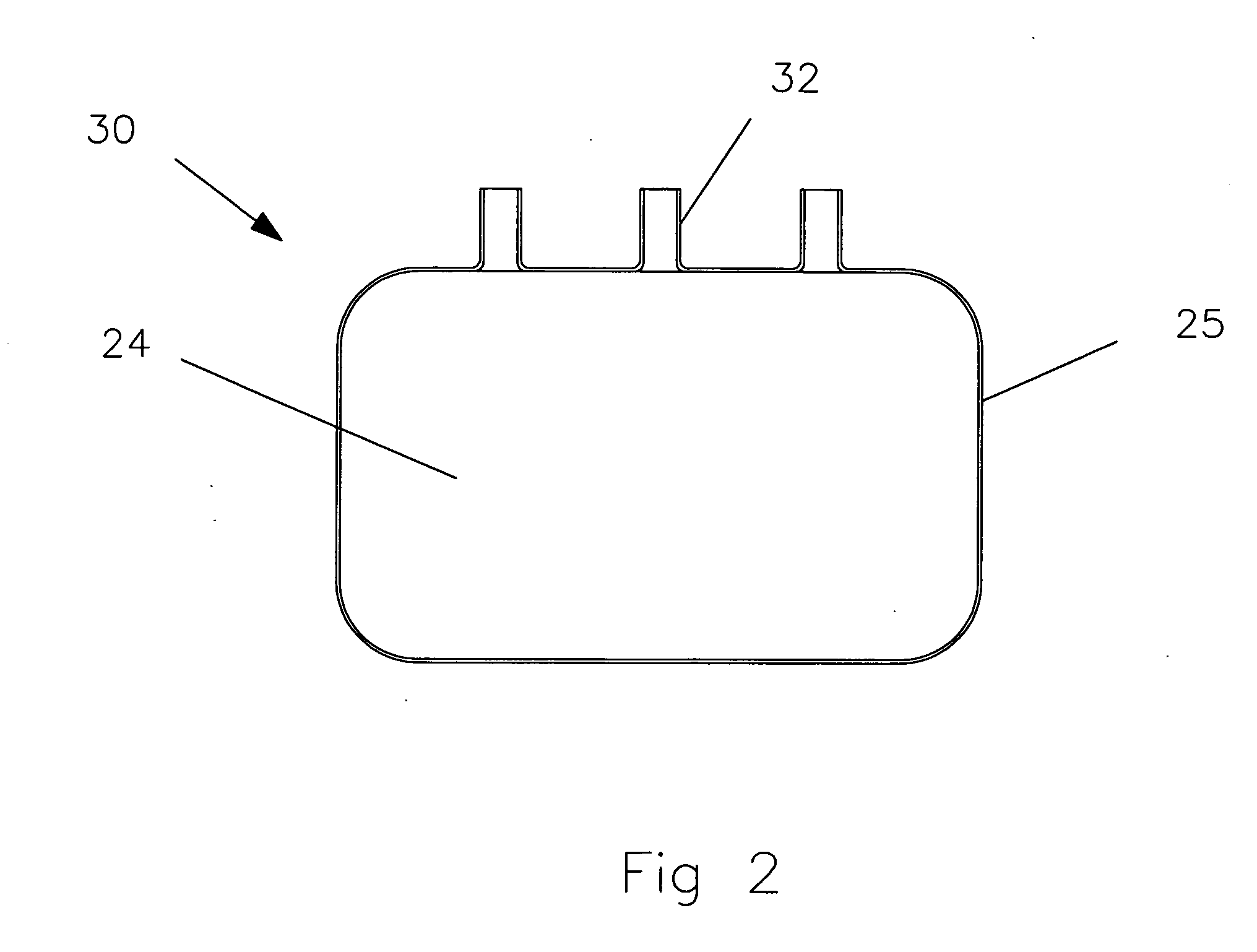
[0033] A top view of a metallic container 20 is shown in FIG. 1. The container is generally having the shape of a rectangular parallelepiped with a large flat base and a relatively little height. The large flat base and top surfaces are referred to as planar surfaces 24. The side su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com