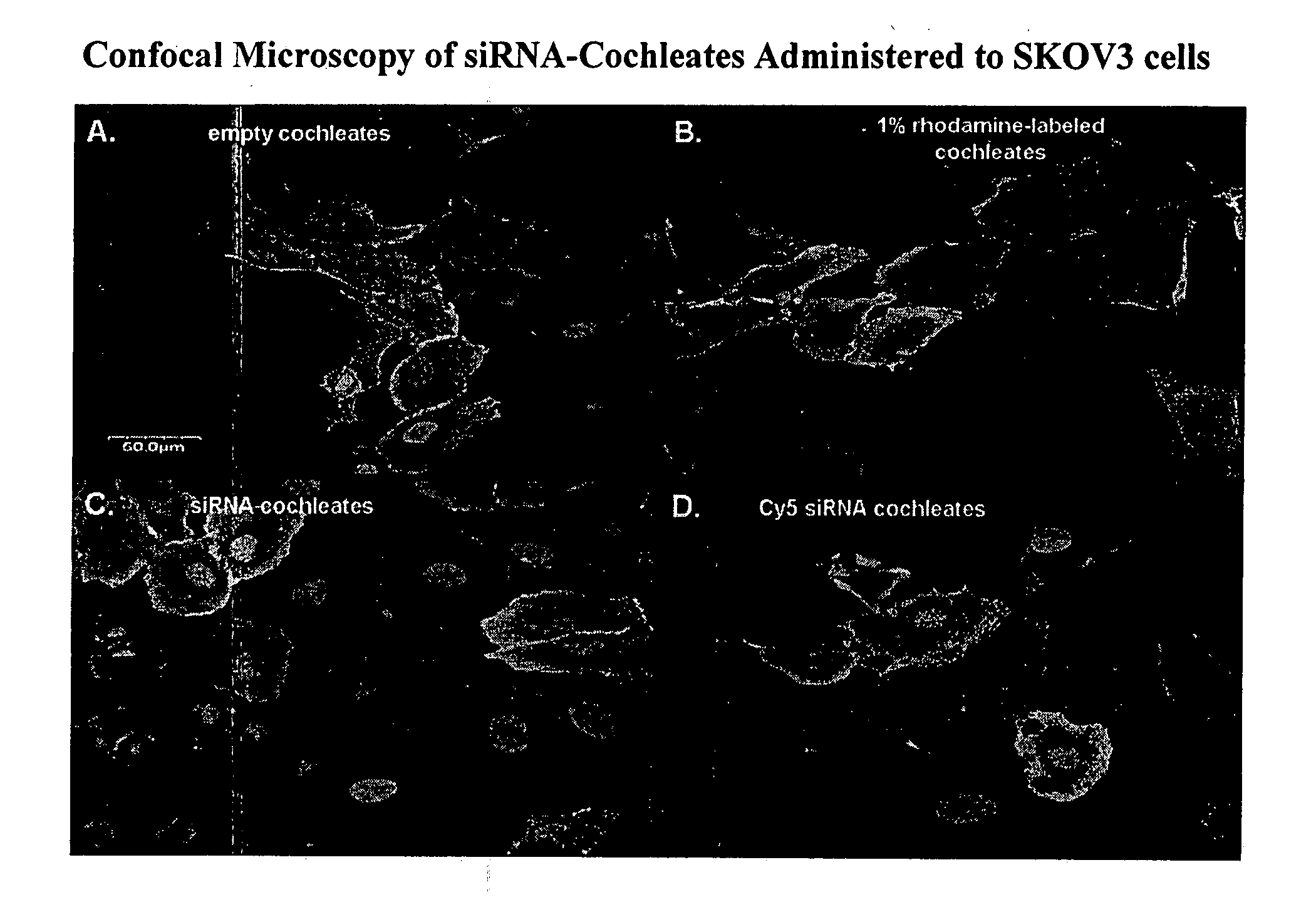
Cochleate compositions directed against expression of proteins
a technology of cochleate composition and protein, applied in the direction of oxidoreductase, peptide/protein ingredients, dna/rna fragmentation, etc., can solve the problems of cytotoxicity, poor cell penetration, and limited use of antisense currently available,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Morpholino Cochleates
[0278] Rhodamine-labeled phosphatidyl ethanolamine (Rho-PE) liposomes were prepared by adding dioleoylphosphatidylserine (DOPS) and Rho-PE at a ratio of 20:1 (Rho-PE:DOPS) to chloroform at a ratio of 10 mg lipid / ml in a 50 ml sterile tube. The concentration of Rho-PE was approximately 0.1% or 0.01% with respect to the DOPS.
[0279] The sample was blown down under nitrogen to form a film. Once dry, the sample was resuspended with TES buffer at a ratio of 10 mg lipid / ml. The liposomes were then passed through a 0.22 μm filter. The homogenous population of rhodamine-labeled liposomes were stored at 4° C. in the absence of light under nitrogen.
[0280] Morpholinos were obtained from GeneTools, LLC (Philomath, Oreg.) for the GAPDH antisense sequence 5′ATCCGTTGACACCGACCTTCACCAT3′ (SEQ ID NO.: 1), and GAPDH mismatch sequence 5′ATCCCTTGAGACCGAGCTTCTCCAT3′ (SEQ ID NO.: 2). These sequences have been used previously to target the first 25 bases of the coding ...
example 2
Delivery of Morpholinos Via Cochleates into Cells
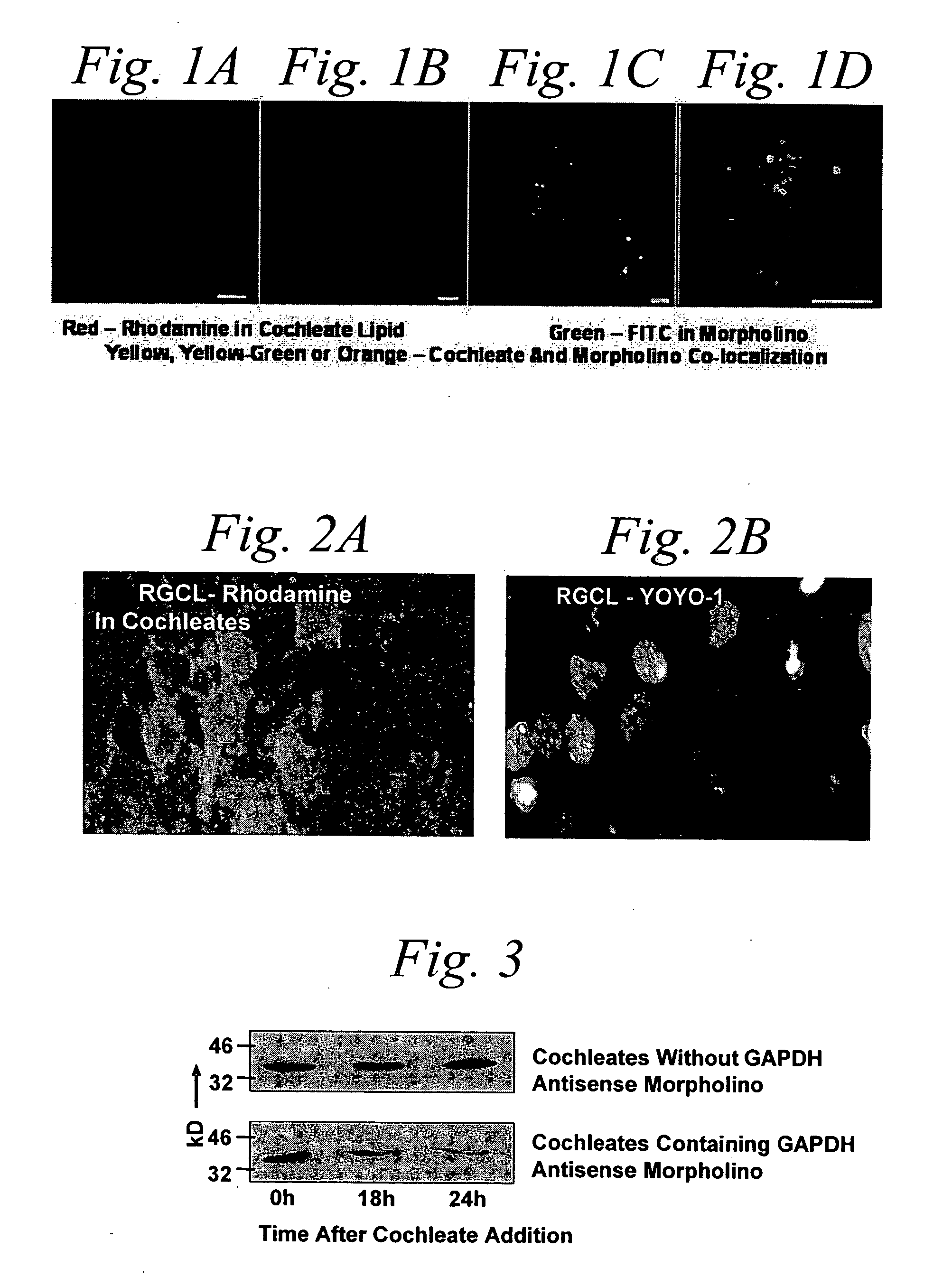
[0284] Morpholino-cochleates were prepared as described in Example 1, with FITC-labeled GAPDH morpholinos. These morpholino-cochleates were administered to NGF differentiated rat P12 cells and photographed at 3 hours and 12 hours as shown in FIGS. 1A and 1B, respectively, after cochleate introduction. As illuminated by the fluoresced rhodamine using LCSM fluorescence imaging, the cochleates fuse with the outer membrane and form submembrane aggregates. FIGS. 1C (low power) and 1D (high power) are photographs of flouresced rhodamine labeled cochleates containing fluorescein isothiocyanate (FITC) labeled morpholinos. FIGS. 1C and 1D depict cochleates containing morpholinos, morpholinos that have been released into the cytosol from unwrapped cochleates, and the delivery of FITC labeled anti-GAPDH Morpholino into the cytoplasm. The morpholinos delivered into the cells depicted in these FIGS. 1A-D were retained in the cells for at least 72...
example 3
Delivery of Morpholinos Via Cochleates Into Retinal Ganglion Cells
[0287] Morpholino-cochleates were prepared as described in Example 1 with FITC-labeled GAPDH morpholinos. These morpholino-cochleates were administered to retinal ganglion cells in situ in retinal organotype culture. It was observed that the morpholino-cochleates readily interacted with the cells in the retinal ganglion cell layer (FIGS. 2A and 2B). FIGS. 2A and 2B are images of X-Y RGCL LCSM computational slices demonstrating avid cochleate uptake by retinal ganglion cells in situ. Scale bars indicate 10 micrometers.
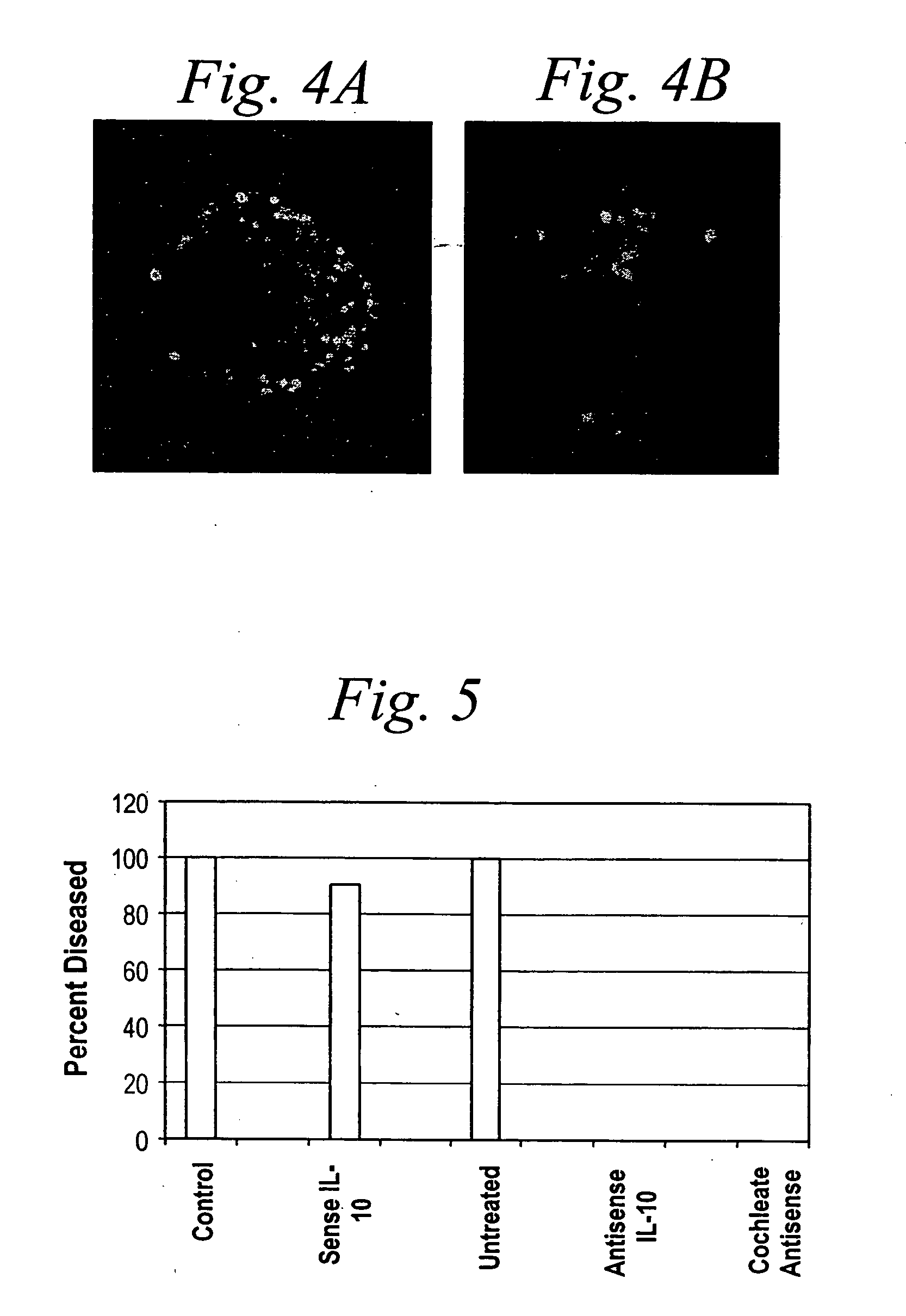
[0288]FIG. 2A indicates cochleate delivery and biological activity of the antisense molecules. Interference with GAPDH by the antisense molecule triggers apoptosis, detected here by YOYO staining of all the retinal ganglion cells in the field. Cell nuclei with apoptotic chromatin condensation have very bright homogeneous YOYO signals (See FIG. 2B). This system provides a very efficient technique for del...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com