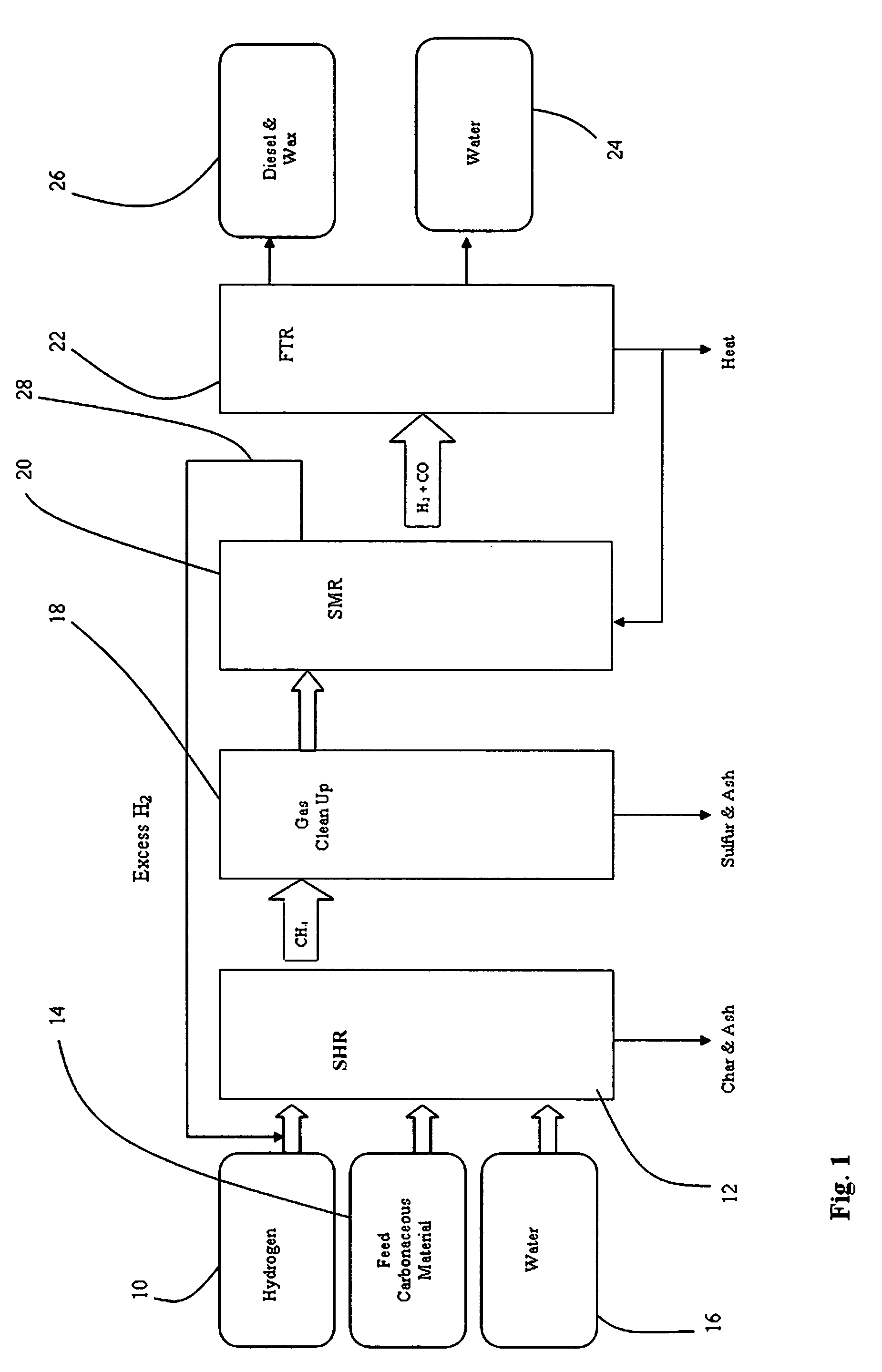
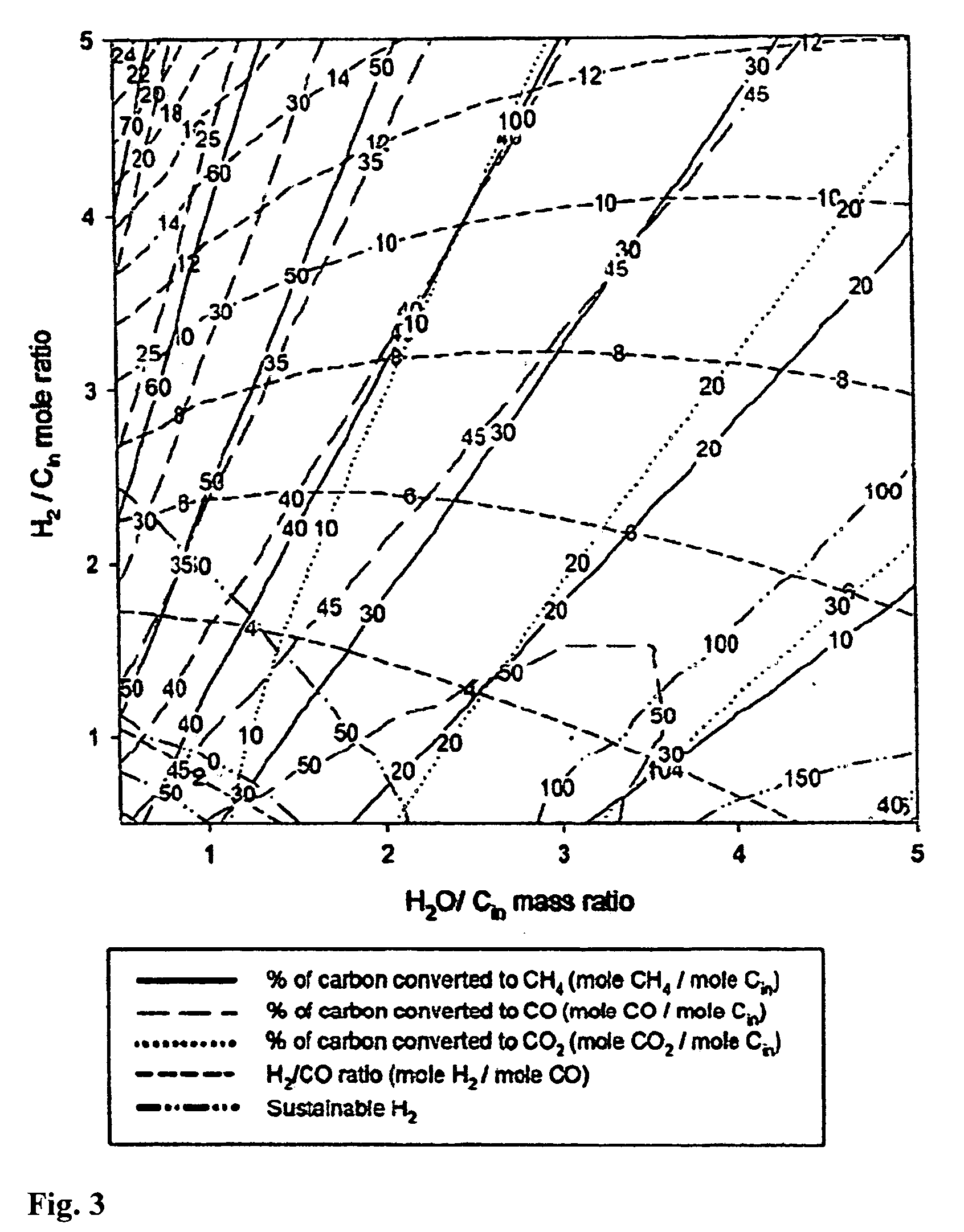
Controlling the synthesis gas composition of a steam methane reformer
a technology of steam methane reformer and synthesis gas composition, which is applied in the direction of combustible gas production, combustible gas purification/modification, waste based fuel, etc., can solve the problems of reducing the catalyst efficiency, serious environmental pollution, and a major dependence on petroleum, so as to improve the effect of economics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
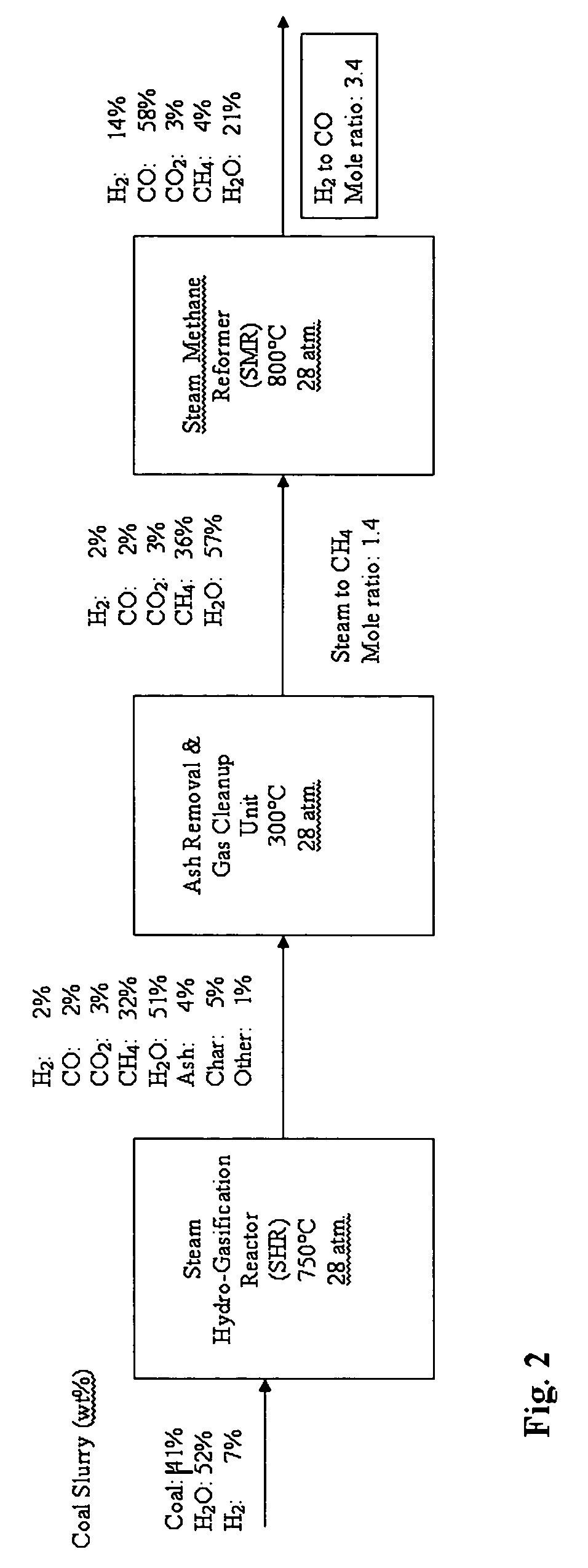
[0031]A mass balance process flow diagram is shown in FIG. 2. The mass percentages of the product stream at each stage of the process are provided in the figure. ASPEN PLUS™ equilibrium process modeling was used to calculate these values. ASPEN PLUS™ is a commercial computer modeling program that allows a process model to be created by specifying the chemical components and operating conditions. The program takes all of the specifications and simulates the model, executing all necessary calculations needed to solve the outcome of the system, hence predicting its behavior. When the calculations are complete, ASPEN PLUS™ lists the results, stream by stream and unit by unit, and can present the data in graphical form with determining ordinate and abscissa
[0032]As shown in FIG. 2, an SHR feedstock of hydrogen and 41% coal slurry results in the production of synthesis gas with a 3.4:l mole ratio of hydrogen to carbon monoxide in the SMR. The required feed hydrogen for the SHR can be supp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mole ratio | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com