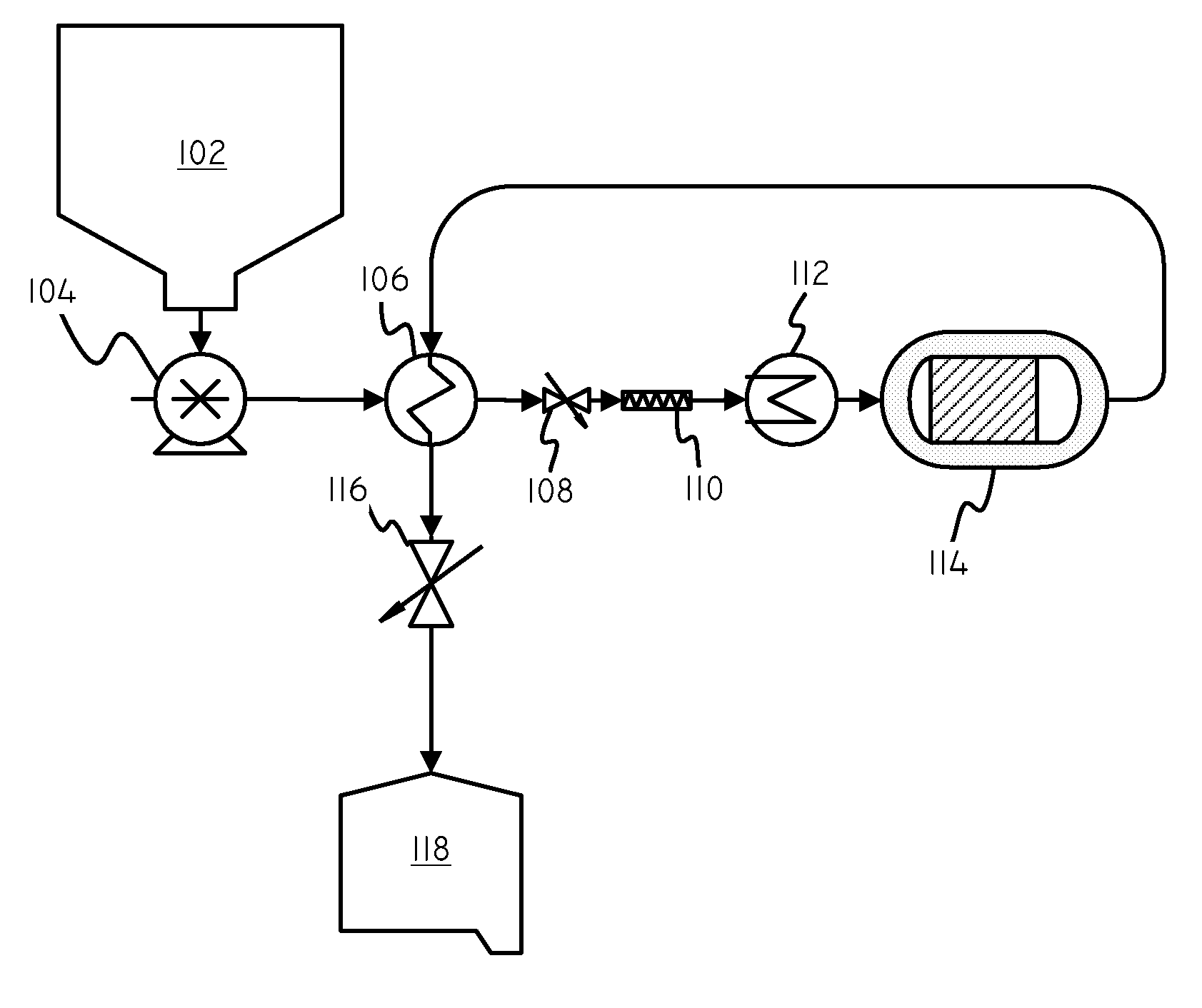
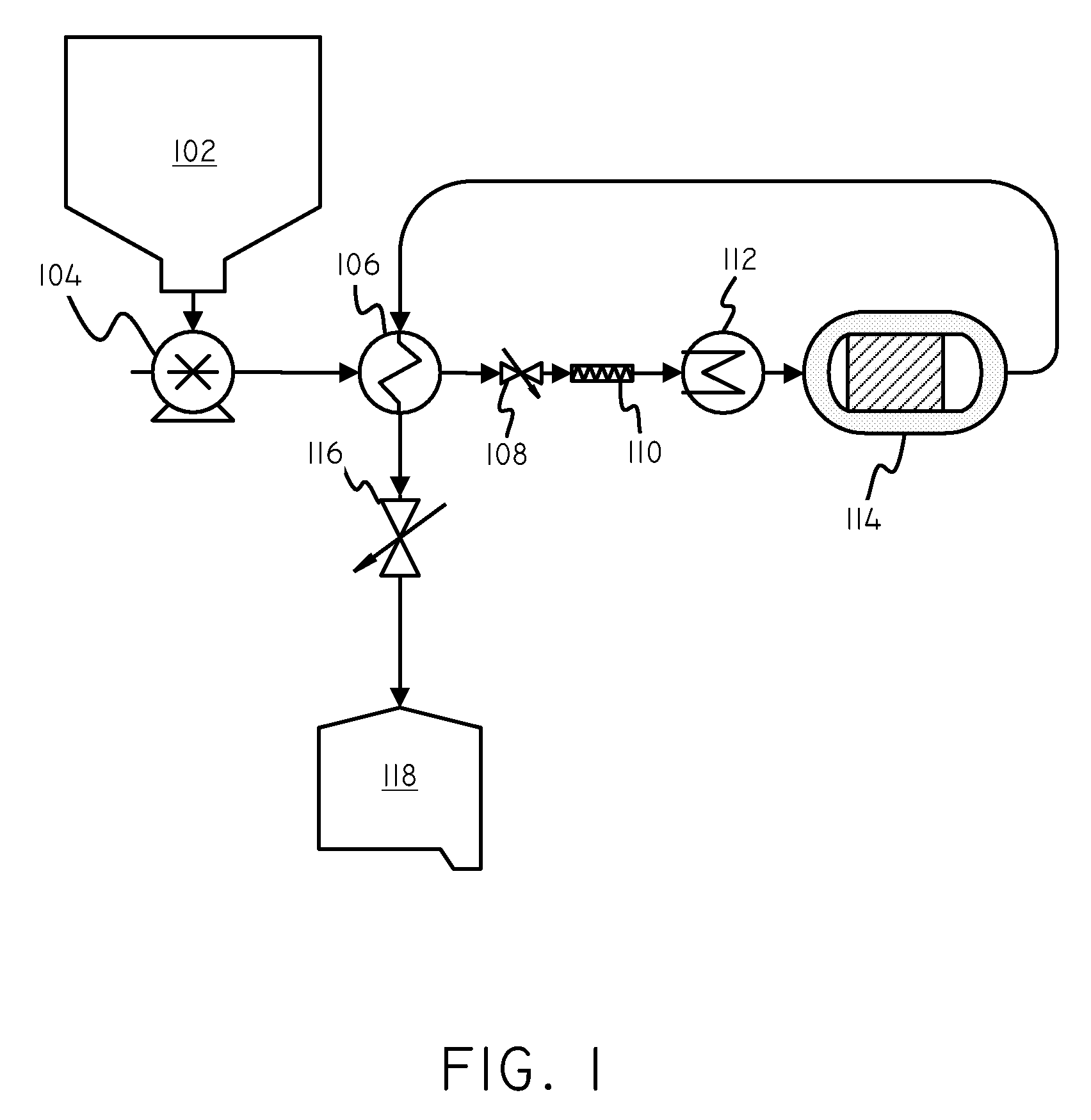
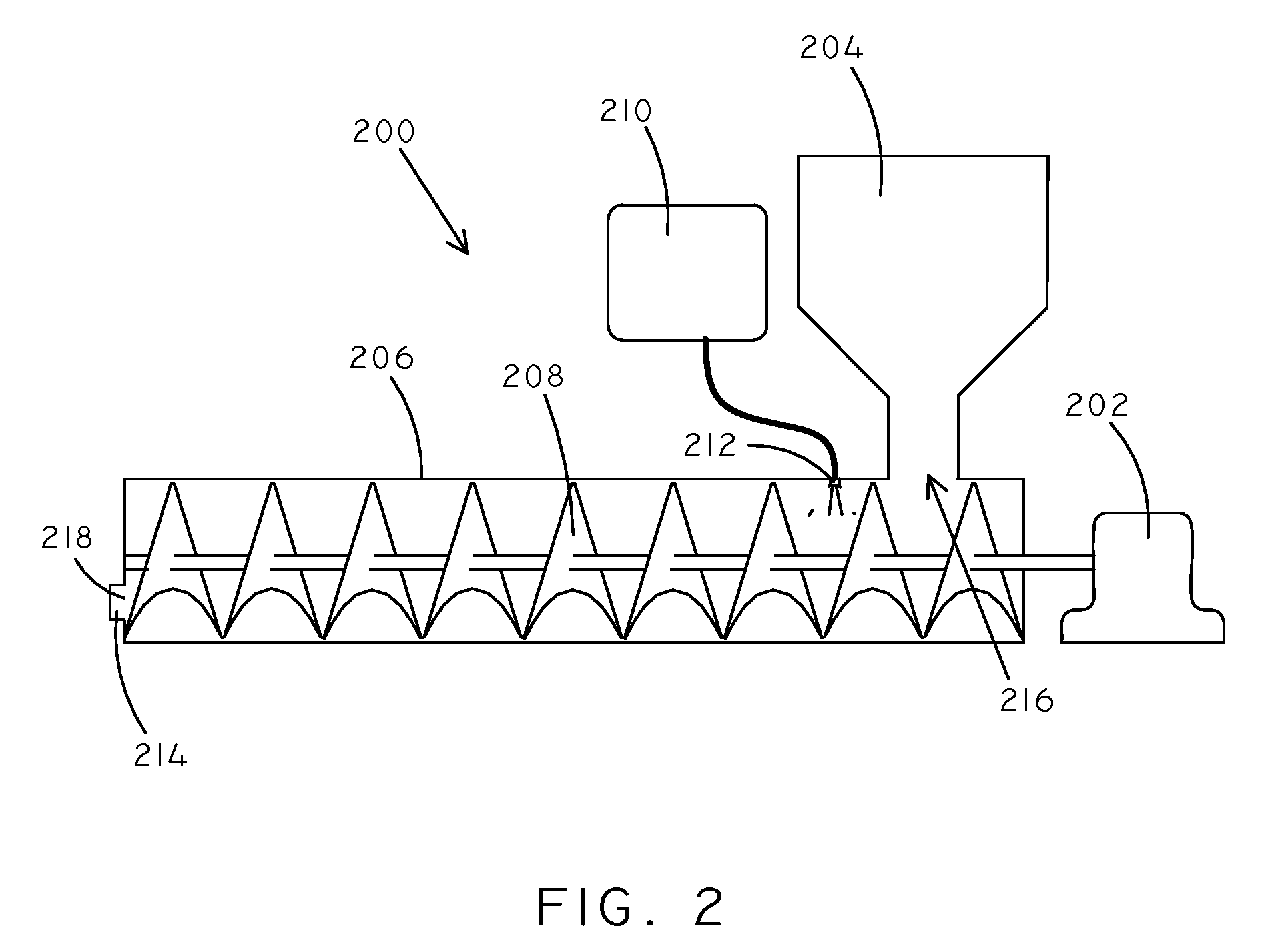
Catalysts and Methods for Complex Carbohydrate Hydrolysis
a technology of complex carbohydrates and catalysts, which is applied in the direction of physical/chemical process catalysts, sugar derivates, organic chemistry, etc., can solve the problems of complex carbohydrates that are not readily metabolized, not as readily usable by living organisms as glucose, and create safety problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Formation of Zirconia Particles
[0063]A colloidal dispersion of zirconium oxide (NYACOL™ ZR 100 / 20) (Nyacol Nano Technologies, Inc., Ashland, Mass.), containing 20 wt. % ZrO2 primarily as about 100 nm particles was spray dried. As the dispersion dried, the particles interacted strongly with one another to provide aggregated ZrO2 particles. The dried aggregated particles that were obtained were examined under an optical microscope and observed to consist mostly of spherules from about 0.5 μm to about 15 μm in diameter.
[0064]The dried spherules were then sintered by heating them in a furnace at a temperature of 750° C. for 6 hours. The spherules were air classified, and the fraction having a size of approximately 10 μm was subsequently isolated. The particles were all washed in sodium hydroxide (1.0 Molar), followed by water, nitric acid (1.0 Molar), water and then dried under vacuum at 110° C. BET nitrogen porosimetry was performed in order to further characterize the sintered spherul...
example 2
Formation of Base Modified Zirconia Particles
[0065]1 liter of 2.0 M sodium hydroxide was placed in a 2 liter plastic Erlenmeyer flask. 110 g of 5-15 μm bare zirconia prepared as described in Example 1 was put into the flask. The particle suspension was sonicated for 10 minutes under vacuum and then swirled for 2 hours at ambient temperature. The particles were then allowed to settle and the alkaline solution was decanted and then 1.4 liters of HPLC-grade water was added to the flask followed by settling and decanting. Then 200 mL of HPLC-grade water was added back to the flask and the particles were collected on a nylon filter with 0.45 micron pores. The collected particles were then washed with 2 aliquots of 200 mL HPLC-grade water followed by 3 aliquots of 200 mL of HPLC-grade methanol. Air was then allowed to pass through the particles until they were free-flowing.
example 3
Formation of a Packed Column
[0066]Particles as formed in Example 3 were slurried in methanol (26 g zirconia in 44 mL of methanol) and packed into a 15 cm×10.0 mm i.d. stainless steel HPLC column at 7,000 PSI using methanol as a pusher solvent. The column was allowed to pack for 8 minutes under pressure and then the pressure was allowed to slowly bleed off and the end fitting and frit were attached to the inlet of the column. 200 mL of total solvent was collected in the packing process.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com