Compounds and methods to enhance rAAV transduction
a technology of enhancing raav and compounding, applied in the introduction of vector-based foreign material, viruses, peptide/protein ingredients, etc., can solve the problems of major obstacles in the trafficking of internalized viruses to the nucleus, endosomal processing and other problems, to achieve the effect of increasing episomal stability or persistence of vectors, and increasing episomal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
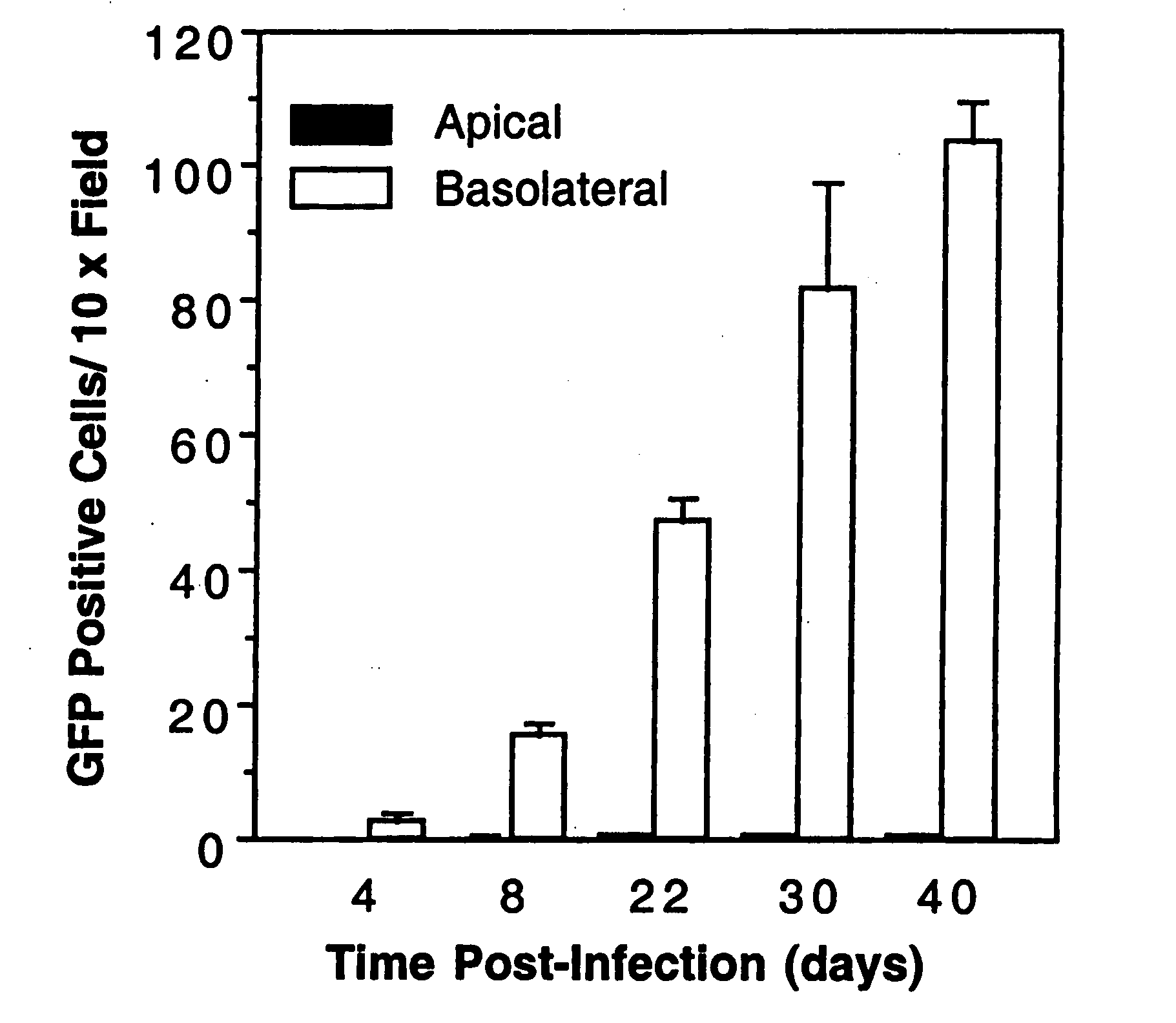
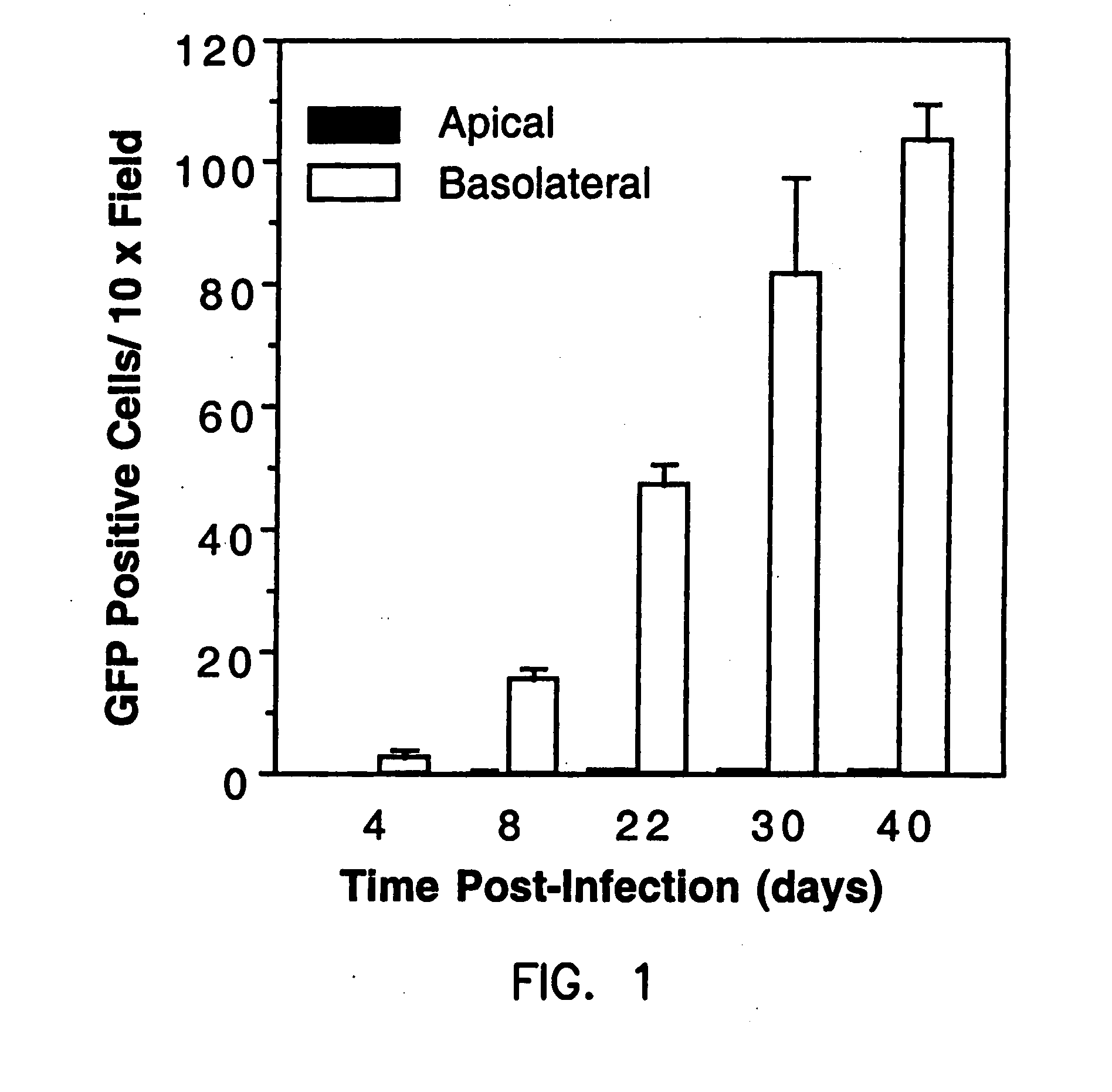
Polarity and Time Course of rAAV Transduction in Bronchial Epithelial Cells Methods
[0196]Primary culture of polarized human bronchial epithelia. Primary human airway epithelial cells were collected by enzymatic digestion of bronchial samples from lung transplants as previously described (Kondo et al., 1991; Zhang et al., 1995). Isolated airway primary cells were seeded at a density of 5×105 cells / cm2 onto collagen-coated Millicell-HA culture inserts (Millipore Corp., Bedford, Mass.). Primary cultures were grown at the air-liquid interface for more than 2 weeks, at which time differentiation into a mucociliary epithelium occurs. The culture medium, used to feed only the basolateral side of the cells, contained 49% DMEM, 49% Ham's F12 and 2% Ultraser G (BioSepra, Cedex, France).
[0197]Production of rAAV. Recombinant AAV virus was produced by a CaPO4 co-transfection protocol and was purified through three rounds of isopycnic cesium chloride ultracentrifugation, as previously described (...
example 2
AAV Binding at the Basolateral Membrane
[0202]Although receptor abundance supports the notion that rAAV binding may be limiting at the apical surface, to conclusively demonstrate this fact requires direct assessment of virus binding. To this end, radiolabeled virus was used to study binding at 4° C. in the absence of endocytosis. In addition, total binding and entry was also studied under the same conditions described above for gene expression studies. Environmental stimuli known to enhance rAAV transduction in other systems (i.e., UV irradiation) were also evaluated.
Methods
[0203]Viral binding and uptake assays. Tritium-labeled AV.GFP3ori was prepared according to a previously published protocol (Summerford et al., 1998) with several modifications. Briefly, methyl-3H thymidine (specific activity: 3159 GBq / mmol, NET-027Z, NEN Life Science Products, Inc. MA) was added to the cell culture medium at a final concentration of 1 μCi / ml at 7 hours post-transfection with pCisAV.GFP3ori and pR...
example 3
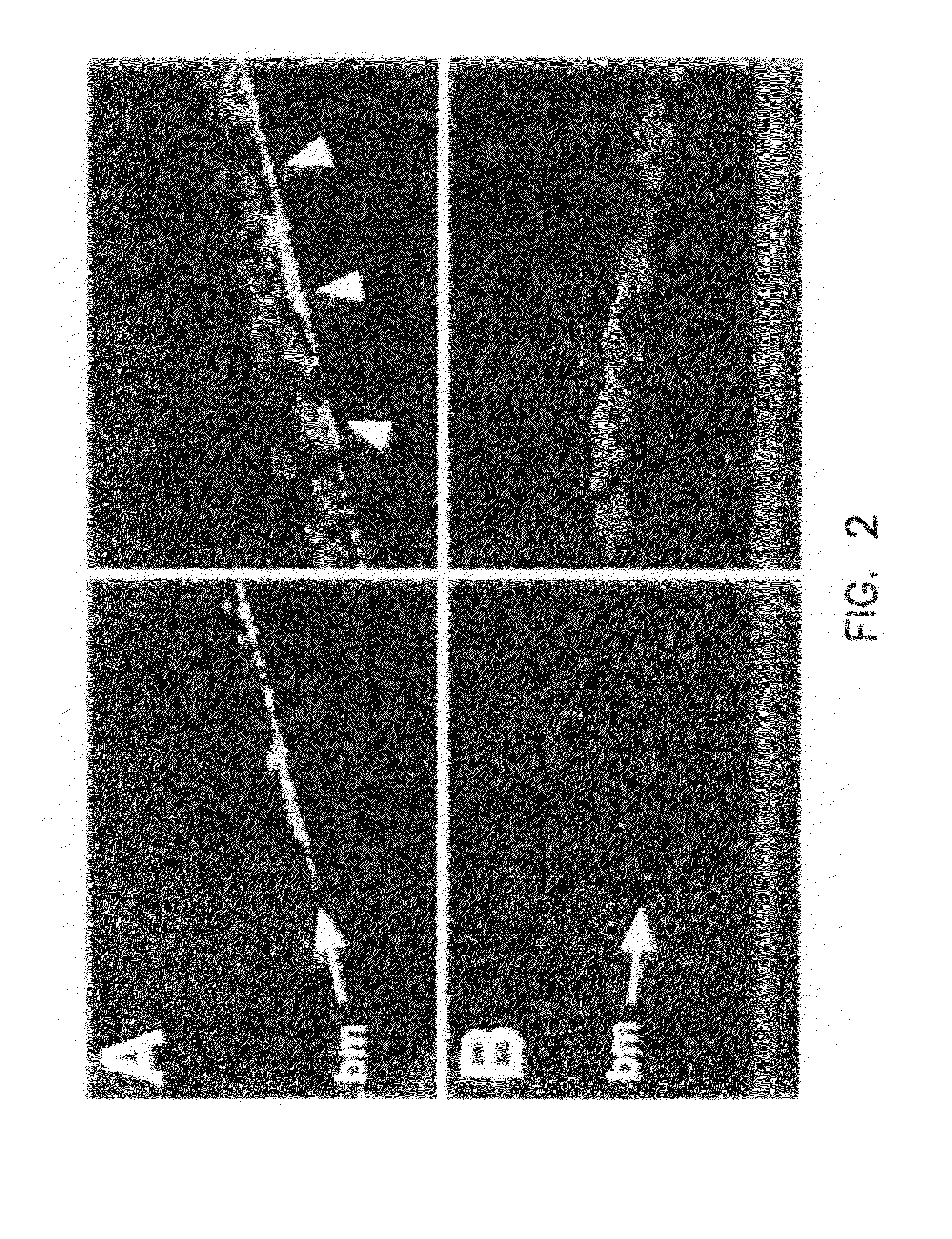
Evaluation of Viral Endocytosis and Trafficking to the Nucleus Using Cy3 Labeled rAAV
[0209]To assess endocytosis and nuclear trafficking of AAV following treatment with various agents which may modulate these processes, fluorescently labeled virus was prepared. Virus was labeled using the following protocol: Three times CsCl banded rAAV (AV.GFP3ori) was conjugated with the bifunctional NHS-ester carbocyanine-Cy3 using a modified procedure from Amersham (Piscataway, N.J.). This procedure conjugates Cy3 to amine groups in the viral capsid as esters linkages. Briefly, 5×1011 particles of the virus were incubated for 30 minutes at 4° C. with increasing concentrations of the NHS-ester carbocyanine-Cy3 due in a reaction volume of 1 ml. Several experimental conditions evaluated crosslinking reactions with dye:rAAV particle ratios of 0.2, 1, 5, 25, 100, and 200. The solution was transferred to a dialysis chamber (10,000 MW cut-off; Gibco BRL, Gaithersburg, Md.) and dialyzed for 24 hours aga...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com