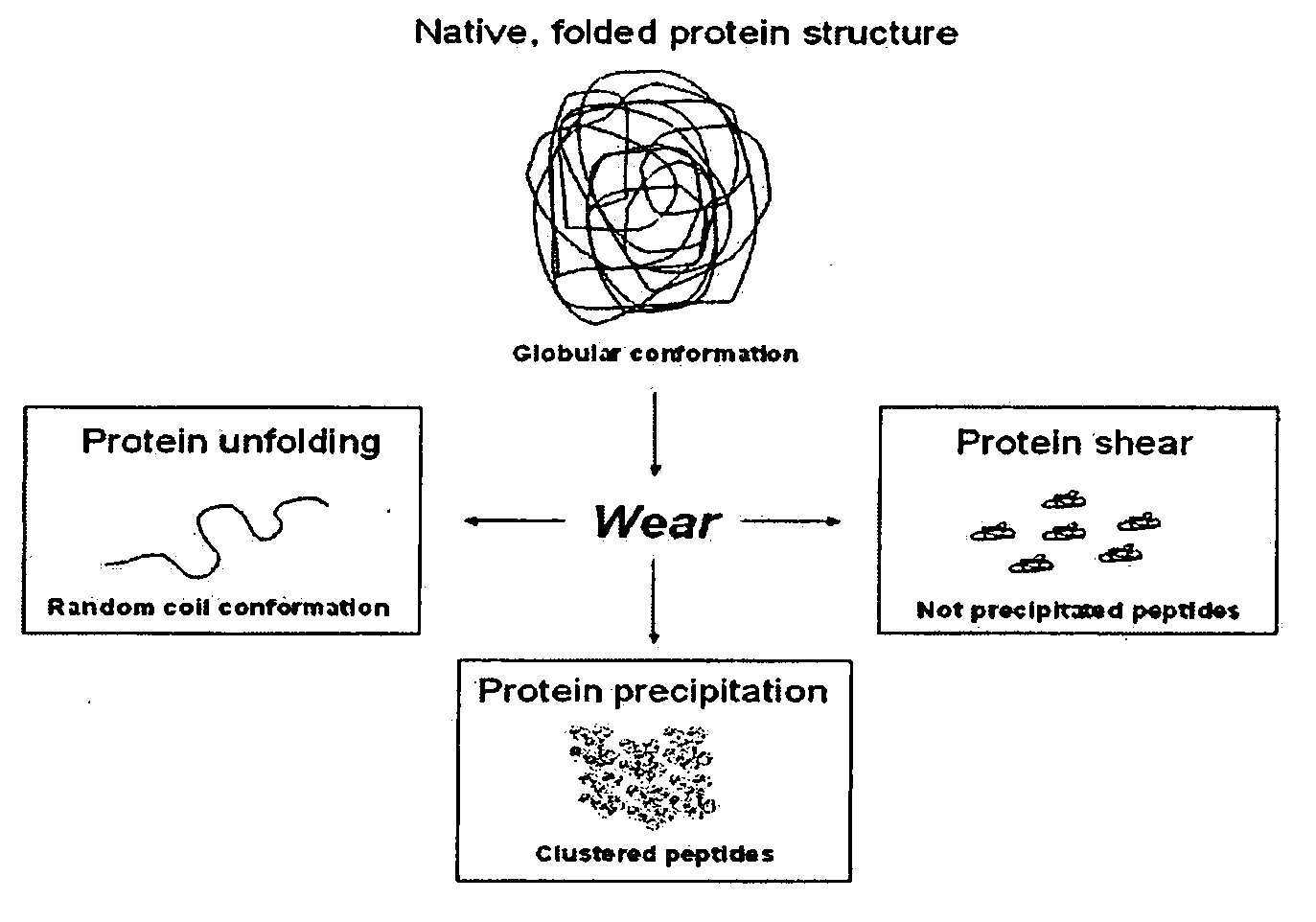
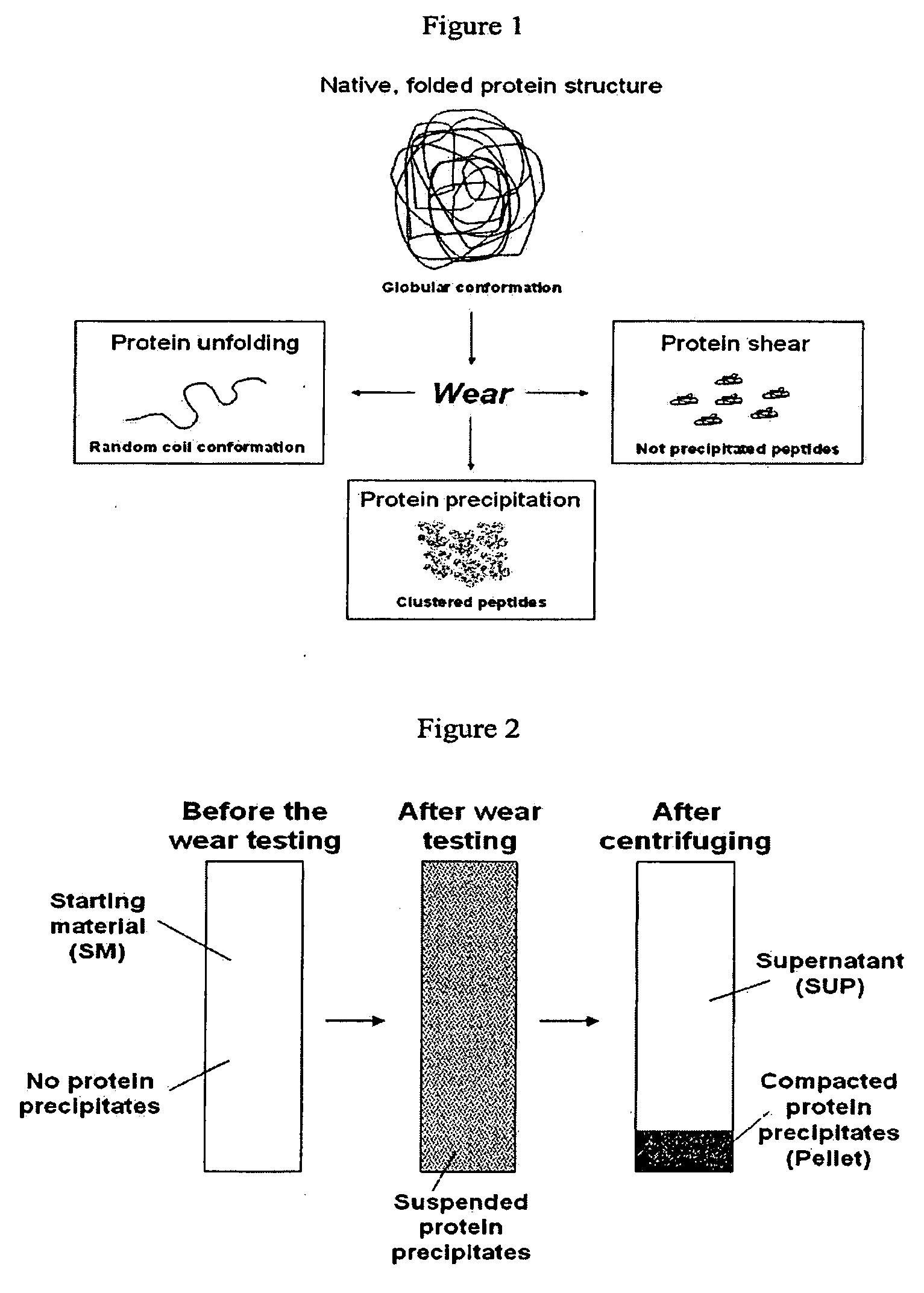
Lubricant for wear testing of joint replacements and associated materials
a joint replacement and lubricant technology, applied in the direction of biochemistry apparatus and processes, enzymes, additives, etc., can solve the problems of affecting the wear and tear of joints, and each of the serum protein constituents is likely to have a unique effect on wear and tear,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
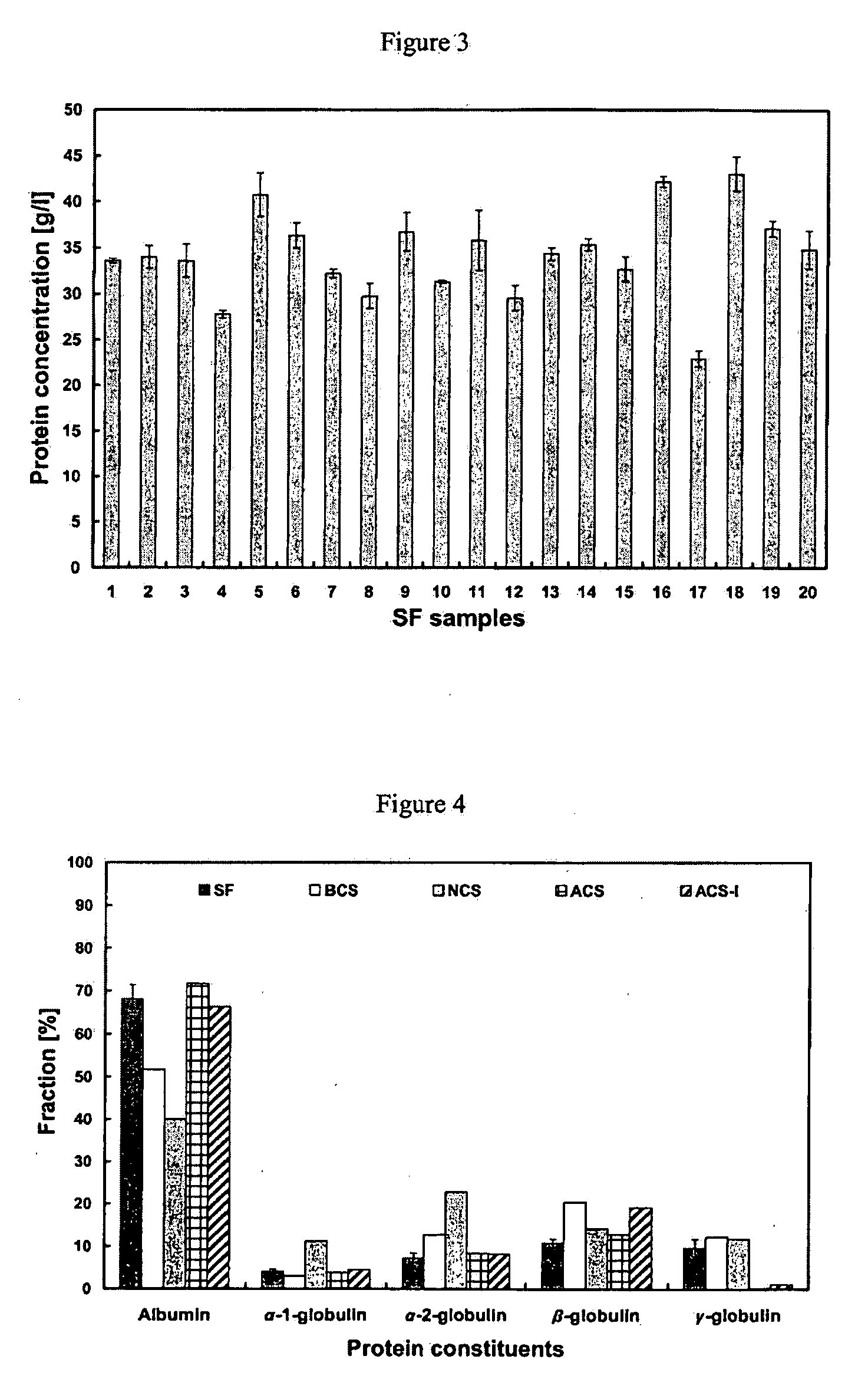
Serum Protein Concentration and Protein Constituent Fractions Vary Depending on the Lubricant
[0072]The ratio of albumin / globulin in serum lubricant at the start of a wear test has been used as an indicator of wear in several hip simulator studies (Wang et al (2004) J. Biomed. Mater. Res. 68B(1):45-52), however, determination of clinically relevant fractions of specific serum proteins, including α-1, α-2, β, and γ-globulins, in wear simulator testing has not been evaluated, nor has the relevance of these protein fractions in wear simulator testing.
[0073]Twenty patients were selected to participate in the present study: ten male and ten female patients, with a mean age of 64.7 years (range, 60-70 years), undergoing surgery for primary total knee arthroplasty (Table 1). Patients who had previously received any knee injections for pain relief (i.e. Cortisone (a steroid hormone) or Synvisc® HYLAN G-F 20 (Genzyme, Cambridge, Mass.)), or patients with rheumatoid arthritis (RA) or other inf...
example 2
Osmolality and Trace Element Concentrations Vary Depending on the Serum Lubricant and Dilution Medium
[0079]Osmolality is a direct measure of the ionic strength of a solution and is a regarded as a systemic, patient specific value (Baumgarten et al. (1985) J. Bone Joint Surg. Am. 67(9):1336-1339). Individual calf sera samples were diluted either with DW or PBS. The choice of dilutive media was suggested to affect solution osmolality given that osmolality has been shown to affect the thermal stability of proteins in solution (Giancola et al. (1997) Int. J. Biol. Macromol. 20(3):193-204) and, thus, might also affect the protein degradation and the PE wear rate in simulator wear testing.
[0080]In the present study, an osmometer (Osmometer 5520, Wescor, Logan, Utah) was used to determine the osmolality of the SF, different serum lubricants and their dilutive media. The osmometer determined the osmolality following the freezing-point depression test strategy at atmospheric pressure. This s...
example 3
Role of Bacterial Growth on Wear Testing
[0084]A six million cycles wear tests was performed on the knee simulator (AMTI, Waltham, Mass.) with AMK implant (FIG. 7) components (Table 4) (DePuy Orthopaedics, Warsaw, Ind.). There calf serum lubricants were used: BCS lubricant (BCS+DW+SA), NCS lubricant (NCS+DW+SA), and ACS lubricant (ACS+DW+SA).
TABLE 4Test protocol from 0-6 Mc.LubricantMcL implantsR implants0-3BCS + DW + SABCS + DW + SA 3-4.5ACS + DW + SANCS + DW + SA4.5-6 BCS + DW + SABCS + DW + SABCS = bovine calf serum;DW = distilled water;SA = sodium azide;ACS = alpha calf serum;NCS = newborn calf serum.
[0085]The wear rates obtained from the testing with the BCS lubricant, NCS lubricant, and ACS lubricant indicated that the fractions of serum proteins in the lubricant had a significant effect on wear rate (FIG. 8). The serum lubricants were found to have wear varying rates where BCS lubricant >NCS lubricant >ACS lubricant.
[0086]Electrophoresis data obtained for serum lubricant sam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tm-cp-max | aaaaa | aaaaa |
| osmolality | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com