Chemodenervating pharmaceutical as anti-inflammatory agent
a technology of chemodenervating pharmaceutical and denervating tissue, which is applied in the field of chemodenervating pharmaceutical as anti-inflammatory agent, can solve the problems of pain or subjective adverse sensory experience, inability to explain certain medical observations regarding the use of chemodenervating agents, and inability to achieve long-lasting action, reduce inflammation, and reduce inflammation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
case i
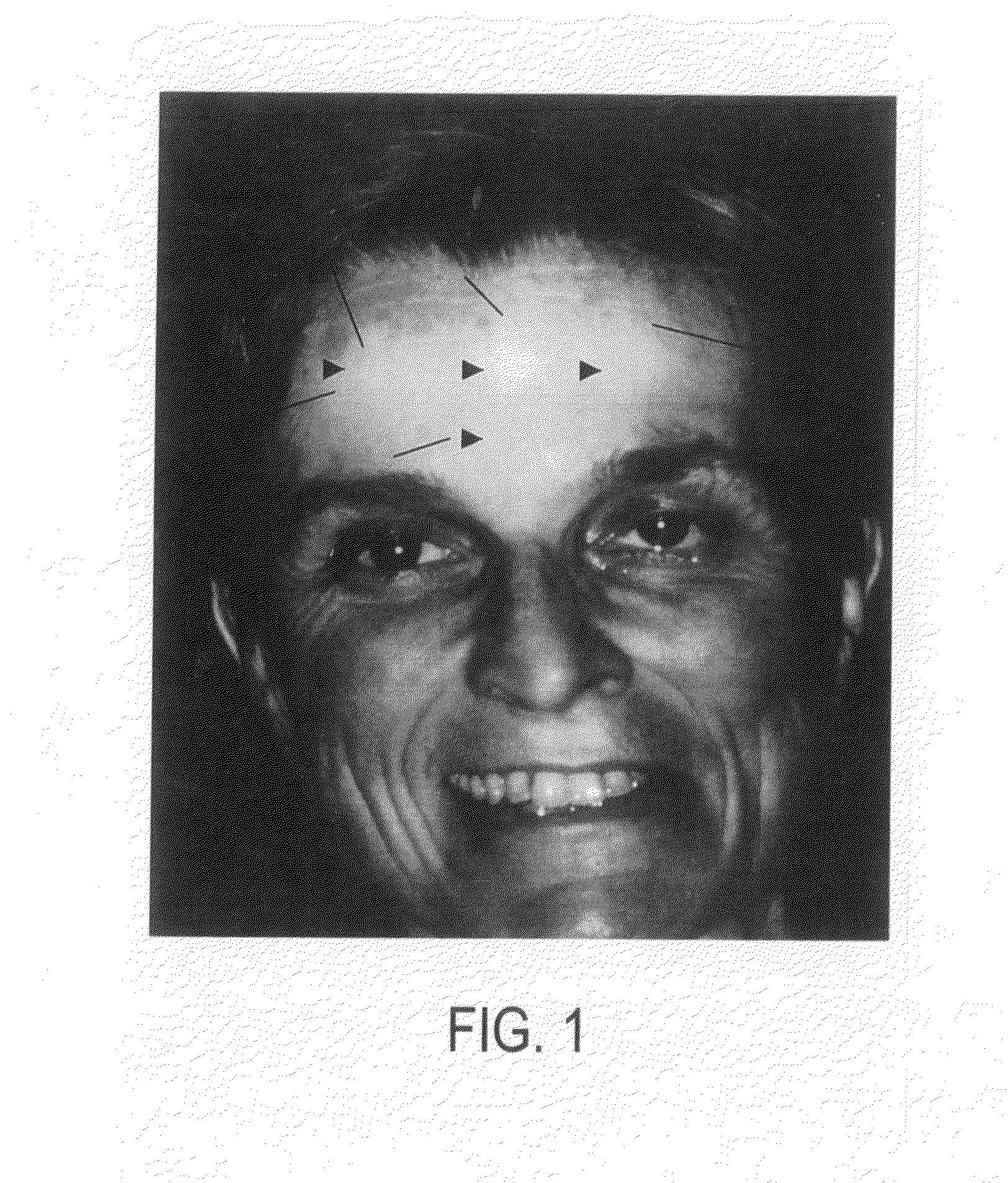
[0036]A 53-year-old woman had a history of Bell's Palsy five years prior to being evaluated for asymmetric facial movements from synkinesis. The facial movements were causing involuntary eyelid closure. Additionally, she noted abnormality in forehead creases and desired achieving facial forehead crease wrinkle symmetry by injection of botulinum into the frontalis muscle. After exercising, she noted that she would traditionally break out in hives, eg. urticaria, throughout her body, with the facial region being most severely involved. This urticarial reaction was closely associated with itching.
[0037]Two weeks after botulinum toxin injection at a dose of 2.5 units per injection site, an unusual phenomenon was noted on her forehead after exercising. An area measuring three to four centimeters around each injection area was protected from hive, itching or erythema. Other areas of the face were involved in the usual hive, erythema and flushing reaction experienced with exercising. The p...
case ii
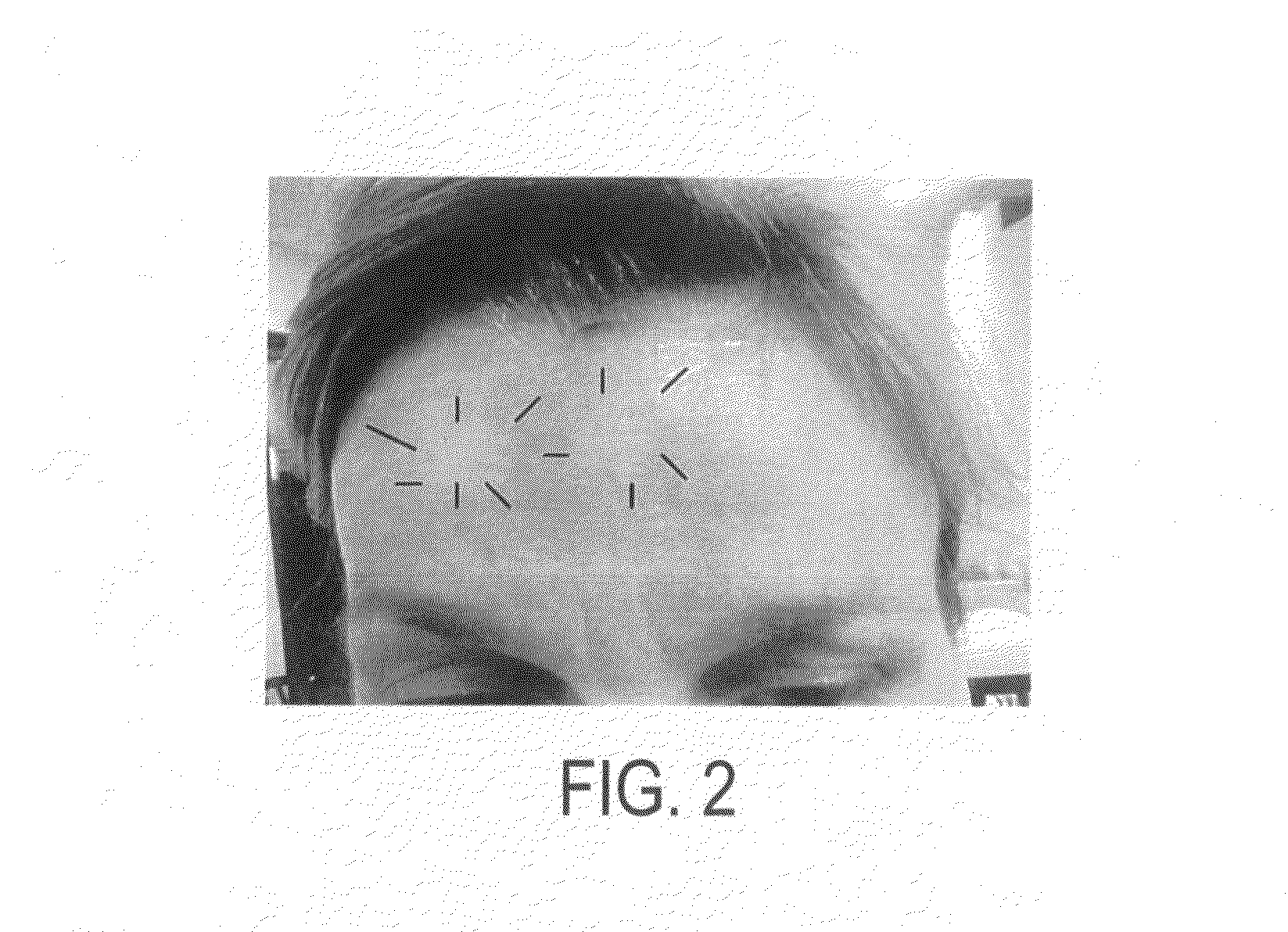
[0040]A 44-year old woman with interest in having glabellar lines reduced with botulinum toxin experienced generally facial flushing and swelling sometimes associated with headache following extreme exertion. After regional botulinum toxin was administered at a dose of 5 units, an area with diameter measuring 25 mm around the injection sites was protected from the flushing and abnormal sensory effect associated with such exertion. She noted this protective effect lasted 10-14 weeks.
[0041]FIG. 2 is a photograph of this woman within three days after injection, showing the blocking effect on heat release, vasodilation, erythema and edema, which effect expands to its maximum in 12 days, and persists for at least four months.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| heat | aaaaa | aaaaa |
| permeability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com