Composition containing arazyme for the prevention and treatment of cancer
a cancer and arazyme technology, applied in the direction of drug compositions, peptide/protein ingredients, chewing gum, etc., can solve the problems that patients with cancers who have been treated by chemotherapy have suffered serious side effects of systemic chemotherapy, cannot be properly treated by surgical operation, and cannot be properly treated by surgery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Arazyme
[0119]To prepare arazyme, the active ingredient of the present invention, Aranicola proteolycius HY-3 (KCTC 0268BP) was cultured in a culture medium (bacto-trypton 0.5%, yeast extract 0.5%, sodium chloride 0.1%, potassium chloride 0.05%, calcium chloride 0.02%, magnesium sulfate 0.02%) at 22° C. for 18 hours. The culture solution was filtered by membrane filtration (2 μm filter, Satorius, USA) to separate supernatant from the cells. The supernatant was concentrated by membrane filtration (10 kDa Membrane filter, Pall sept, PALL Corporation, USA). Arazyme of the invention has the characteristics of anion. So, the concentrated solution was purified by ion exchange resin (Sigma USA) using DEAE-cellulose (Sigma, USA) pre-treated with 50 mM tris-HCl buffer (pH 7.6) and gel filtration exchange resin using Sephadex G-75 (Sigma USA) pre-treated with 20 mM tris-HCl buffer (pH 7.6). The purified enzyme solution was electrophoresed on 10% SDS-PAGE (Sodium dodecyl sulfate-...
example 2
Arrangement of Experimental Mice and Administration
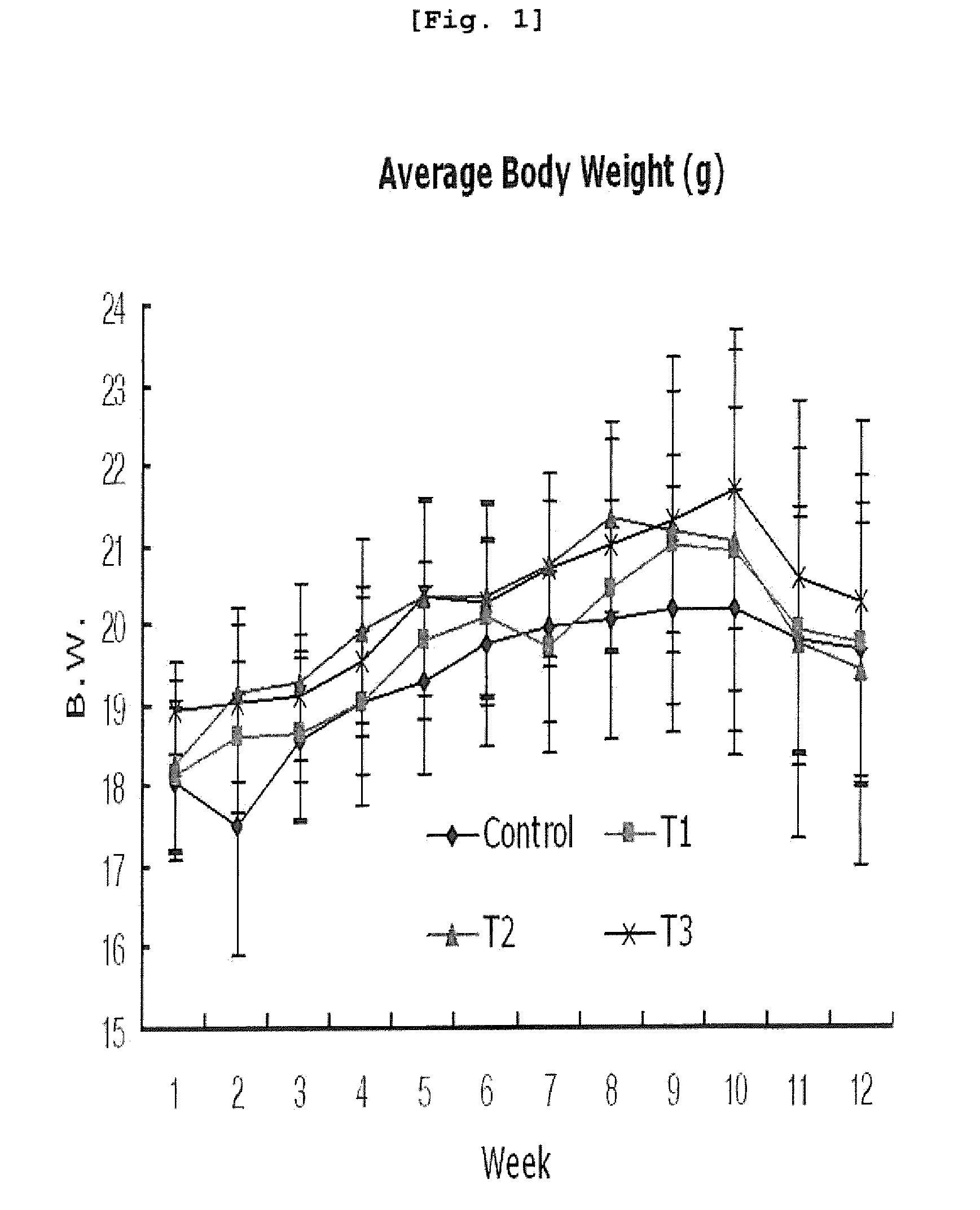
[0120]5-week old specific pathogen (SPF) free CAnN.Cg-Foxnlnu / CrljBgi line nude mice (15 g, Orient, Korea) were used in this invention.
[0121]The mice were inspected and adapted for 7 days in an animal laboratory. During the adaptation, general symptoms were observed and only healthy animals were selected for the experiment.
[0122]The mice were adapted and raised in an animal laboratory equipped with automatic temperature / humidity regulator by which temperature was set at 22±3° C. and the relative humidity was regulated to be 55±10% and light interval was set at 12 hours (light on at 08:00 and light off at 20:00). The environmental conditions were checked regularly (once every three months). From environmental checkup, any environmental changes that might affect the experiment did not detected. During the whole experimental period, the mice were accommodated less than 5 per each polycarbonate cage [240 W×390 L×175 H (mm)]. For individ...
example 3
Cell Culture
[0125]In the present invention, an estrogen receptor negative human breast cancer cell line MDA-MB-231 (HTB-26) provided from ATCC (American Type Culture Collection, USA) distributed from ATCC was used.
Cell Culture Conditions
[0126]The human breast cancer cell line (MDA-MB-231) was subcultured every 2-3 days in DMEM supplemented with 10% FBS (fetal bovine serum) and 1% ampicillin in a 37° C. 5% CO2 incubator. Sterilized culture dishes were used for the experiment.
Arazyme Treatment
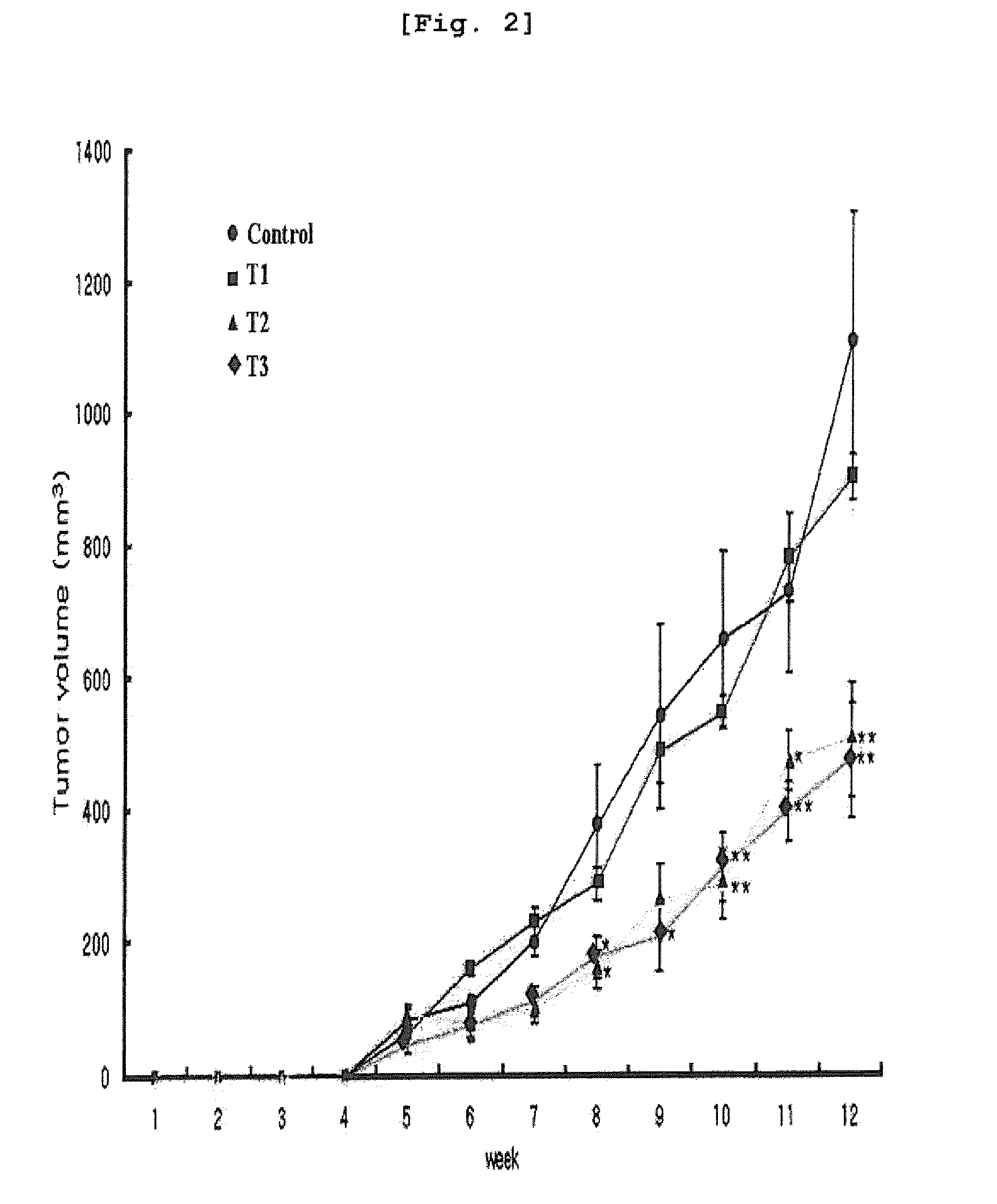
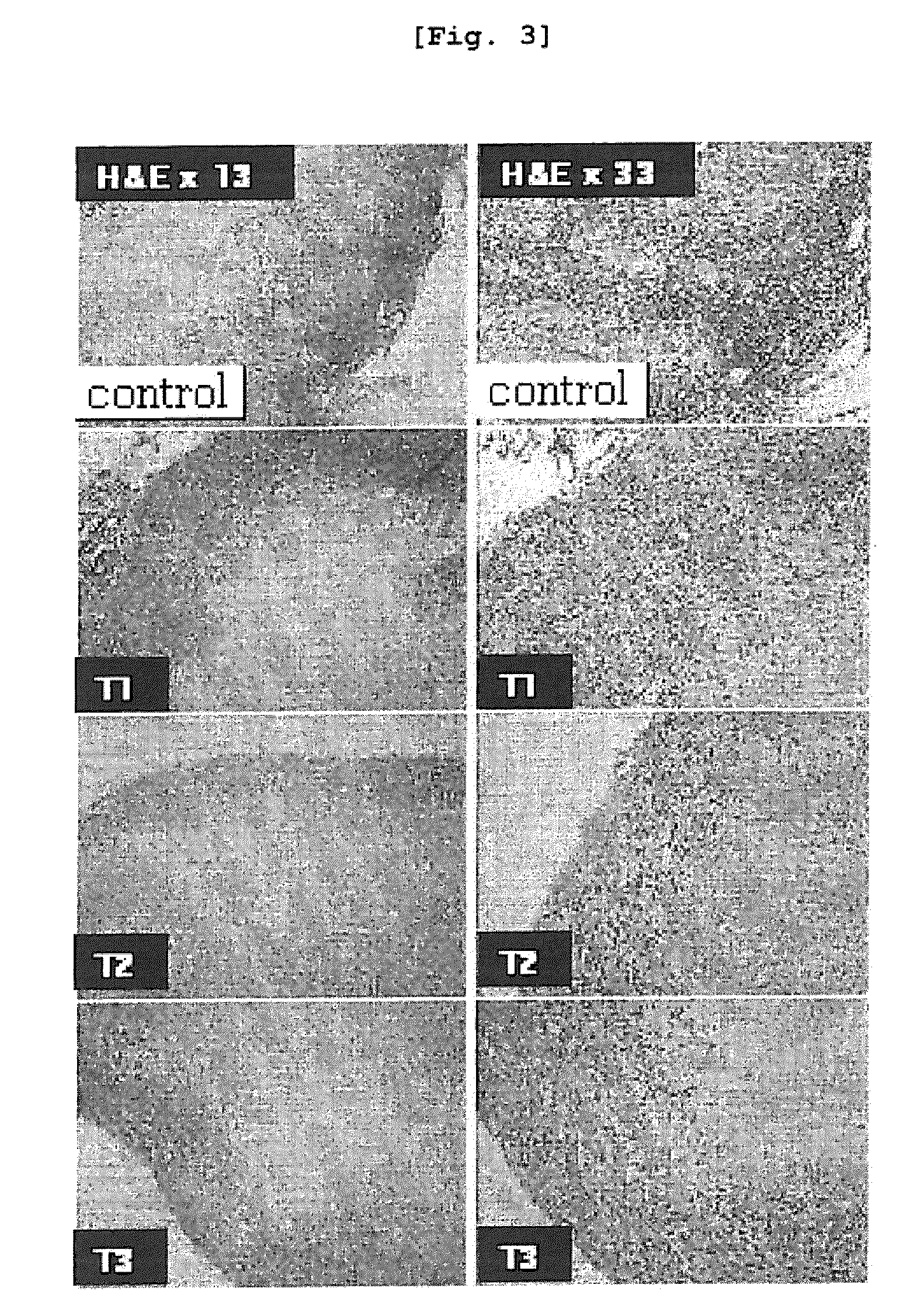
[0127]The human breast cancer cell line (MDA-MB-231) was inoculated in a 6-well dish (6×105 cells / well), followed by culture at 37° C. for overnight. The medium was replaced with a starvation medium containing 1% FBS 4 hours before arazyme was treated, resulting in cell cycle arrest. Arazyme was diluted in sterilized injectable solution at the concentration of 60 μg / ml, which was treated to the culture dish. The culture dish was cultured again in a 37° C. incubator for further 34 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com