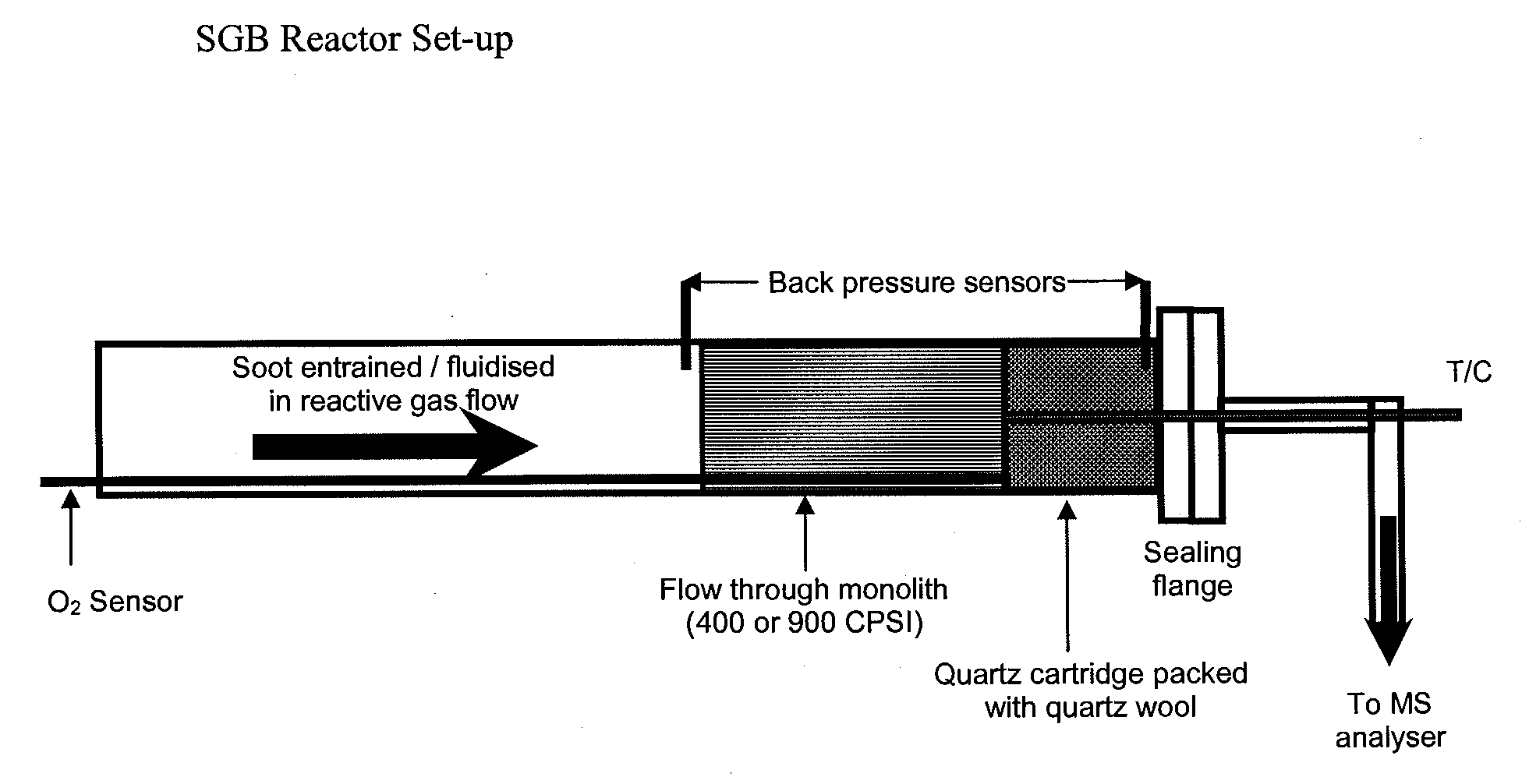
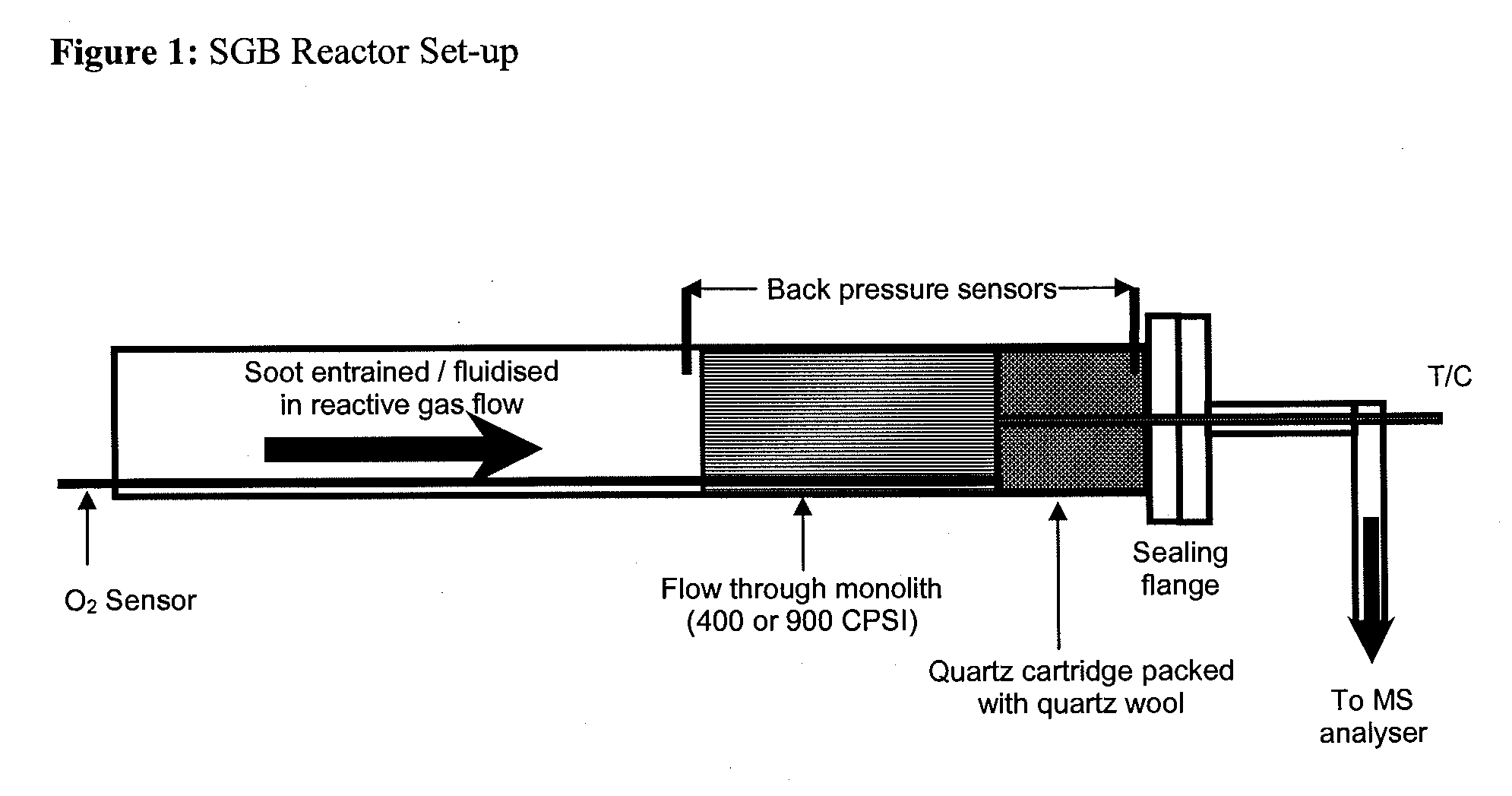
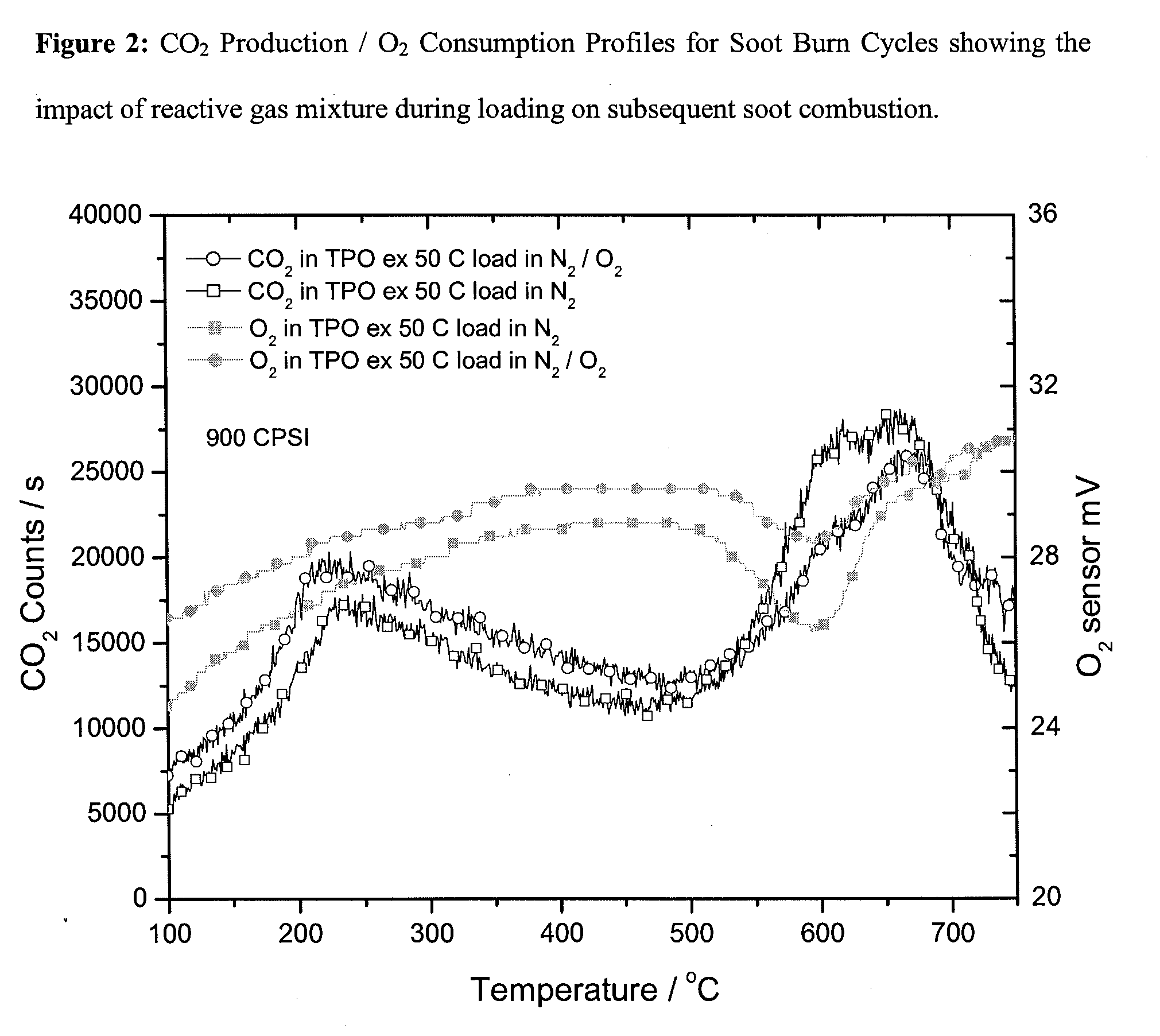
Continuous diesel soot control with minimal back pressure penatly using conventional flow substrates and active direct soot oxidation catalyst disposed thereon
a technology of active direct soot oxidation and continuous diesel, which is applied in the direction of physical/chemical process catalysts, metal/metal-oxide/metal-hydroxide catalysts, and separation processes, etc. it can achieve the effect of facilitating rapid oxidation of soot, further chemical synergies and performance advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0096]The procedure for making 100 grams of 2% Ag(NH3)2 OS, as employed in the test technology is as follows:
[0097]1. Weigh 100 g of OS, correct for moisture content (ca. 1.5% water).
[0098]2. Weigh 3.15 g of silver nitrate crystals. One must compensate for the percentage of metal in the nitrate salt or solution used. Silver nitrate is 63.52% silver.
[0099]3. Dissolve silver nitrate in 50 g deionised water. The amount of water used is determined by the water adsorption capacity of the mixed oxide used. This is generally between 0.5 and 0.5 g water per gram mixed oxide.
[0100]4. Add concentrated NH4OHaq (30% ammonia) to the silver nitrate solution, dropwise, until a clear silver diamine solution is obtained. Solution will first turn brown-black, then clear upon excess addition of ammonium hydroxide.
[0101]5. Add silver diamine solution to mixed oxide powder. Mix thoroughly to produce homogeneous and even-colored moist powder.
[0102]6. Allow powder to rest at room temperature for one hour....
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com