Novel inhibitors of the EGFR kinase targeting the asymmetric activating dimer interface
a dimer and inhibitor technology, applied in the field of molecular biology, biochemistry and cell biology of the epidermal growth factor receptor (egfr), can solve the problems of ineffective pharmaceuticals for many egfr-related illnesses, inefficient process for most drug discovery uses, and interference of egfr kinase activity of these proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Expression and Purification of the Kinase Domain
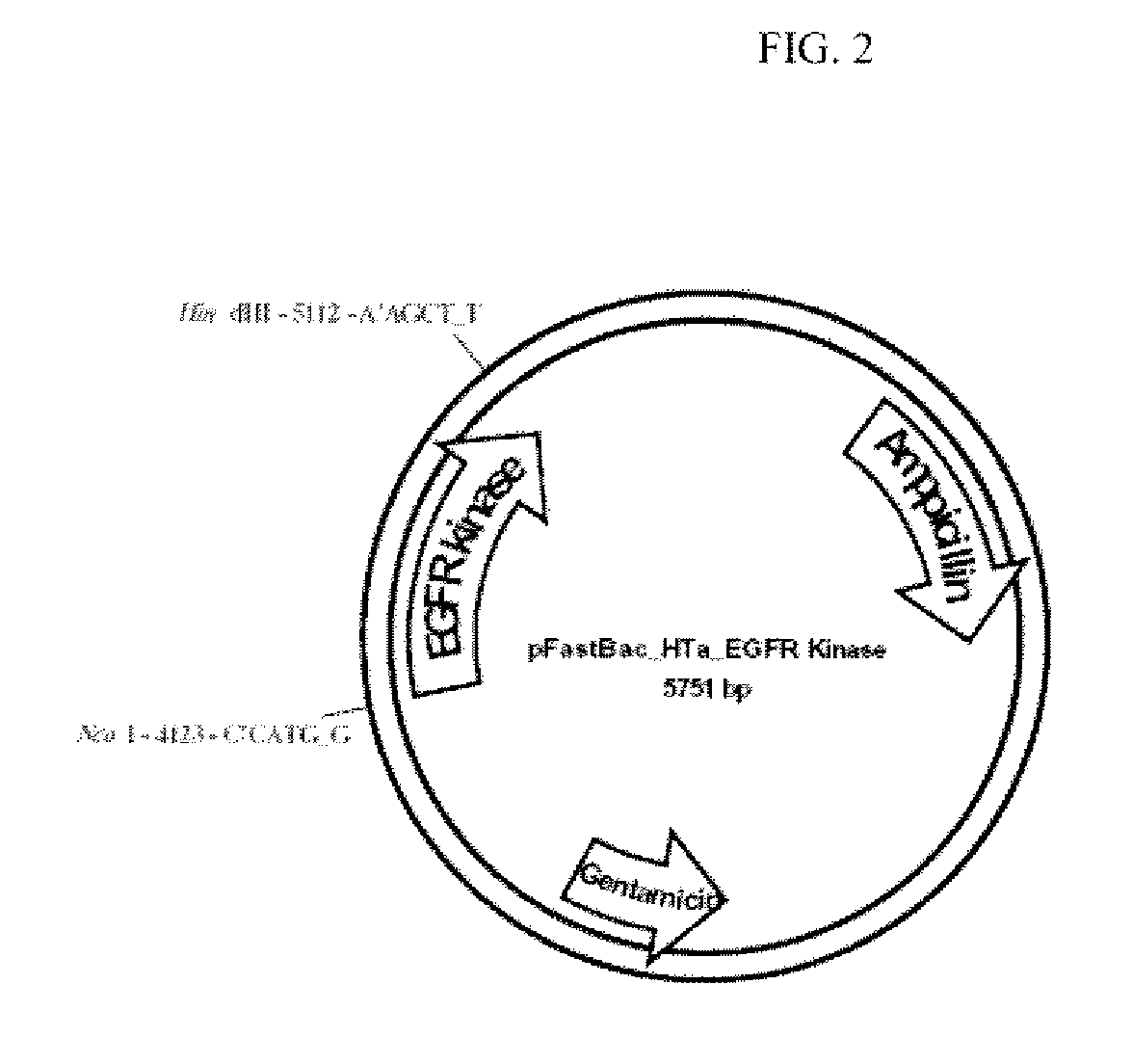
[0218]DNA encoding residues 672-998 of human EGFR was cloned into pFAST BAC HT (Invitrogen) using the NcoI and HindIII restriction sites (FIG. 2). The construct contains an N-terminal 6-His tag, a linker, and a cleavage site for the Tobacco Etch Virus protease (TEV). (MSYHHHHHHDYDIPTTENLYFQGAM). All mutations were introduced using the Quik-change site-directed mutagenesis kit (Stratagene). Sequences of all plasmids were confirmed by DNA sequencing.
[0219]Recombinant bacmid (Bac-to-Bac expression system, Gihco BRL) were transfected into Sf9 cells grown in suspension. Cells were harvested 2-3 days after infection by centrifugation at 4000×g and resuspended in a buffer containing 50 mM Tris, 5% glycerol. 1 mM DTT, and protease inhibitor cocktail (Roche), pH 8.0.
[0220]Cells were homogenized by French press in resuspension buffer and the lysate was centrifuged at 40000×g for 45 minutes. The supernatant was then loaded onto a 60 ml Q-Sepharos...
example ii
Preparation of Small Unilamellar Vesicles
[0222]DOPC and DOGS-NTA-Ni lipids in chloroform (Avanti Polar Lipids, Inc) were mixed in a glass tube. A lipid film was formed upon removing chloroform under a stream of argon gas, followed by putting the tube under vacuum for at least 3 hours.
[0223]Rehydration buffer (10 mM MgCl2, 20 mM Tris, pH 7.5) was added to the lipid film and incubated for at least three hours. Intermittent vigorous vortexing during the incubation was applied to convert the lipid film into large, multilamellar vesicles.
[0224]The multilamellar vesicles were then forced through a polycarbonate filter (pore size: 100 nm) 21-41 times using a mini extruder (Avanti Polar Lipids, Inc) to yield homogenous small unilamellar vesicles.
[0225]The diameter of the vesicles was measured by static light scatting to be in a range from 100-200 nm. (FIG. 16).
example iii
Kinase Assay in Solution and with Vesicles
[0226]A continuous enzyme-coupled kinase assay was performed to measure the kinase activity of the proteins as described in Barker et al., ((1995) Biochemistry, Vol. 34(54): 14843-51), with modifications, as described herein. The ATP concentration was kept to 0.5 mM.
[0227]The buffer used contained 10 mM. MgCl2, 20 mM Tris, and pH 7.5. Replacement of MgCl2 by MnCl2 in the assays resulted in a substantial increase of the catalytic activity of the kinase domain, as noted previously (Mohammadi et al., (1993) Biochemistry (34):8742-8.; Wedergaertner and Gill, (1989) Journal of Biological Chemistry 264(19):11346-53). The substrate peptide was derived from the region spanning Y1173 in EGFR (TAENAEYLRVAPQ). All proteins used in this assay contained the N-terminal (is)6 tag unless otherwise noted.
[0228]The protein concentrations of the EGFR kinase domain used in the assay ranged from 3.5 to 14 μM. The total concentration of the DOGS-NTA-Ni in the bul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Catalytic activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com