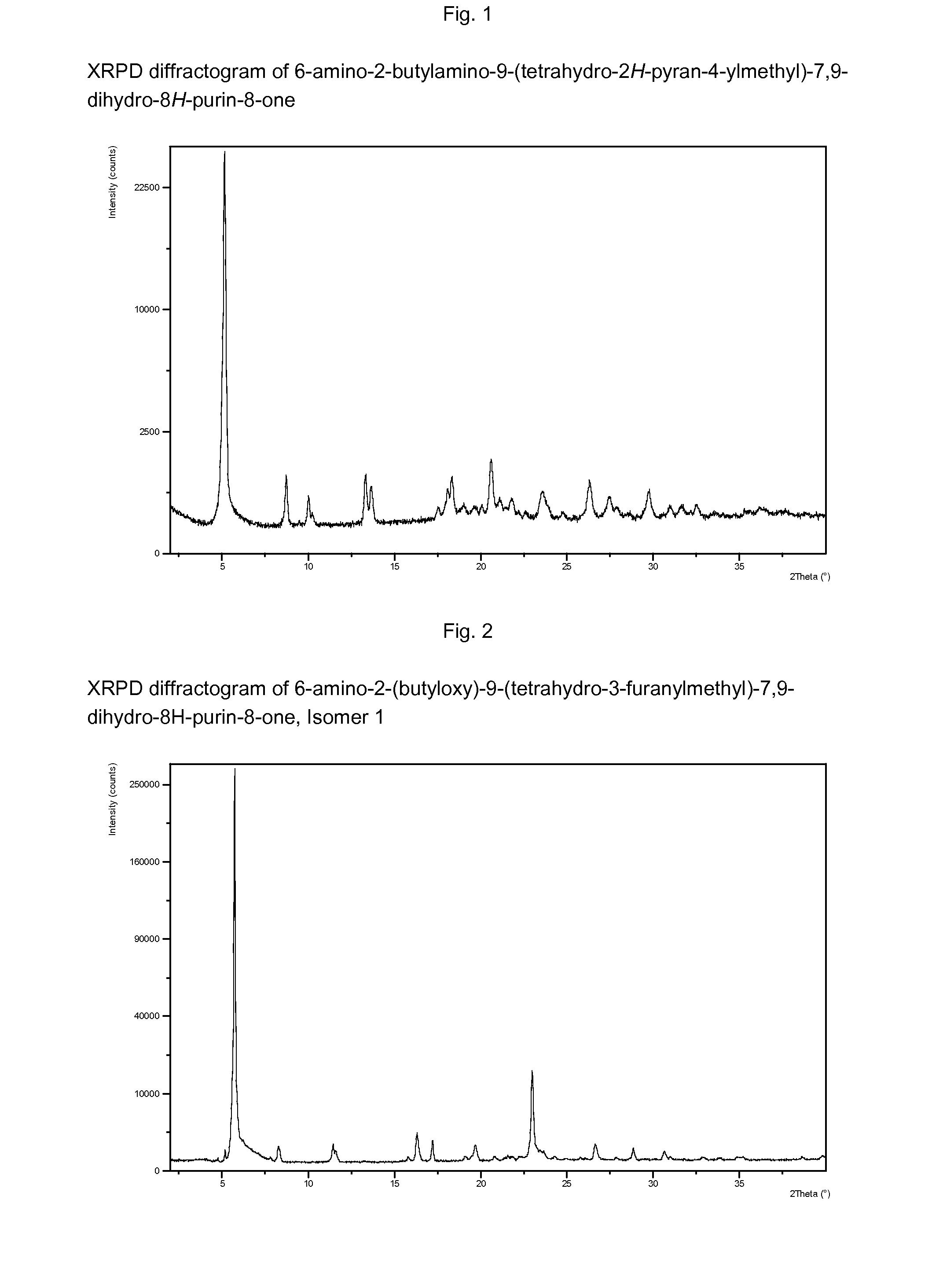
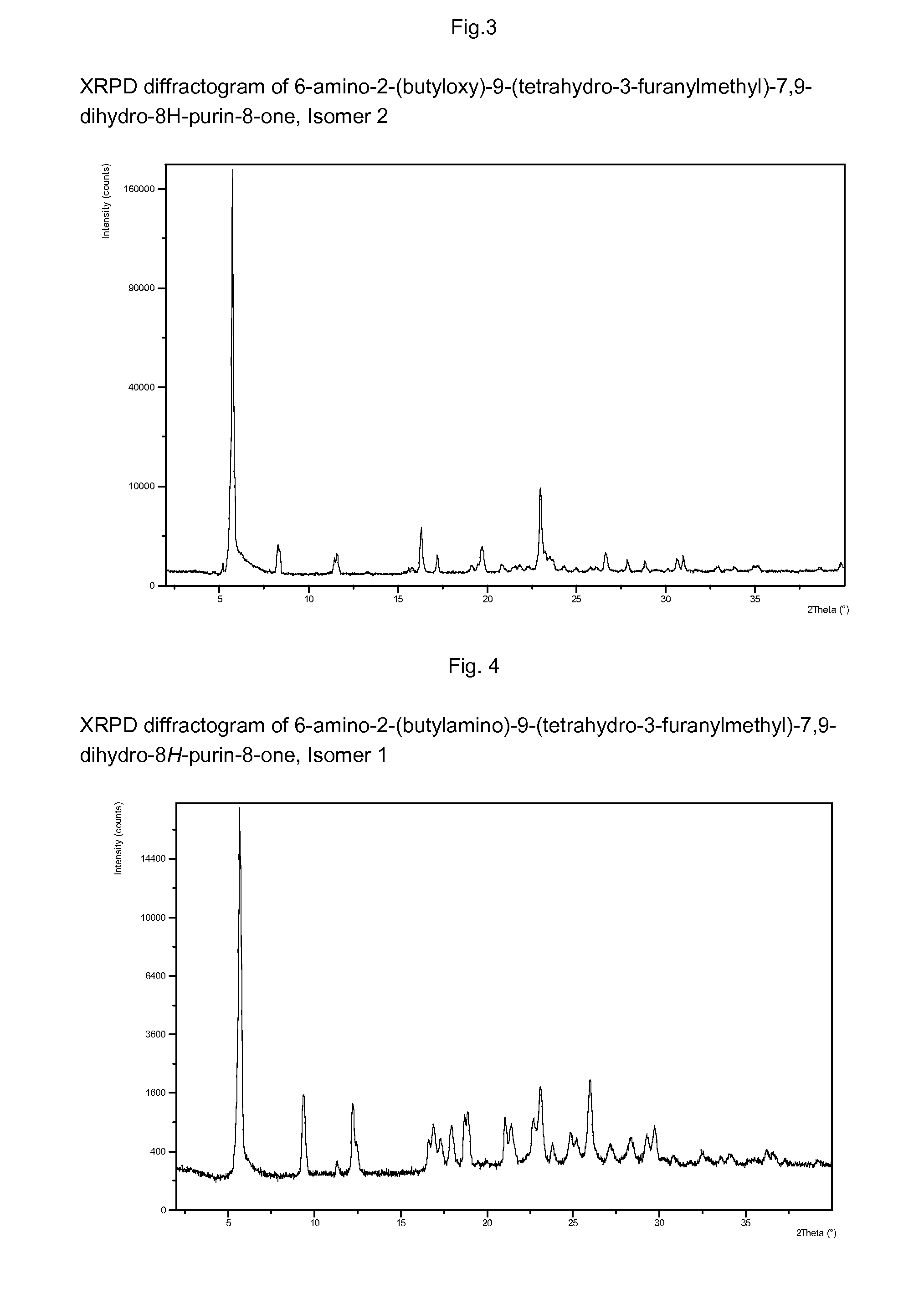
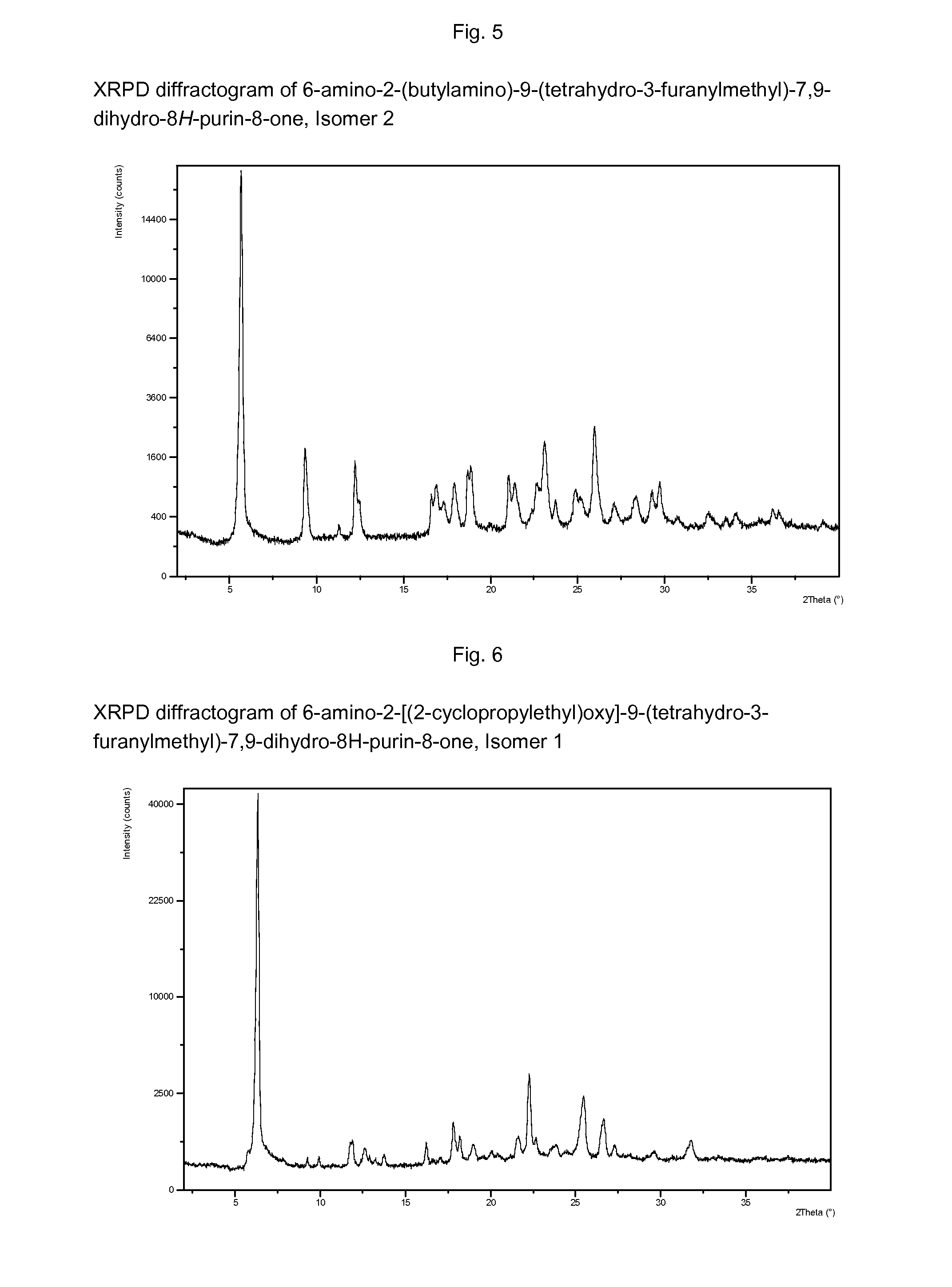
Purine derivatives as immunomodulators
a technology of purine derivatives and immunomodulators, applied in the field of compounds, can solve the problems of inability to show a sustained viral response and inability to control viral load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
6-Amino-2-butoxy-9-(tetrahydro-2H-pyran-4-ylmethyl)-7,9-dihydro-8H-purin-8-one
[1591]
[1592]2-Butoxy-8-methoxy-9-(tetrahydro-2H-pyran-4-ylmethyl)-9H-purin-6-amine (0.22 g) was dissolved in methanol (10 mL) and treated with 4N hydrogen chloride in 1,4-dioxane (1 mL). The reaction was stirred at room temperature 16 hours and stripped to give a solid that was suspended in water (2 mL) before sufficient methanol was added until a solution was obtained. 2N Sodium hydroxide solution was added to bring to pH 7, and the solution concentrated until a suspension formed. The white solid was filtered and washed with water (2 mL, twice to complete transfer and wash). This was dried under suction and then under vacuum at 50° C. to give the title compound as a white solid (0.189 g).
[1593]MS calcd for (C15H23N5O3)+=321
[1594]MS found (electrospray): (M+H)+=322
[1595]1H NMR ((CD3)2SO): 9.86 (1H, s), 6.41 (2H, s), 4.14 (2H, t), 3.81 (2H, m), 3.55 (2H, d), 3.22 (2H, m), 2.03 (1H, m), 1.64 (2H, m), 1.49-1....
example 2
6-Amino-2-butoxy-9-(tetrahydro-2H-pyran-2-ylmethyl)-7,9-dihydro-8H-purin-8-one
[1596]
[1597]2-Butoxy-8-methoxy-9-(tetrahydro-2H-pyran-2-ylmethyl)-9H-purin-6-amine (6 mg, ˜85% pure) was dissolved in methanol (1 mL), treated with 4N hydrogen chloride in 1,4-dioxane (0.5 mL) and stirred for 5 hours at room temperature. The mixture was stripped to dryness to give the title compound (77.8:10.8 by LCMS) as a colourless gum (8 mg).
[1598]MS calcd for (C15H23N5O3)+=321
[1599]MS found (electrospray): (M+H)+=322
[1600]1H NMR (CD3OD): 4.54 (2H, t), 4.06-3.65 (5H, overlapping m), 1.87-1.83 (3H, overlapping m), 1.69 (1H, d), 1.52 (4H, overlapping m), 1.35 (2H, m), 1.01 (3H, t) (NH2 & NH protons exchanged).
example 3
6-Amino-2-butoxy-9-(tetrahydrofuran-2-ylmethyl)-7,9-dihydro-8H-purin-8-one
[1601]
[1602]To 2-butoxy-8-methoxy-9H-purin-6-amine trifluoroacetate salt (0.20 g) in dry DMF (5 mL) was added anhydrous potassium carbonate (0.315 g). This was heated to 60° C. for 1 hour and then cooled to room temperature. Tetrahydrofurfuryl bromide (65 uL) was added and the reaction mixture was then heated to 50° C. overnight. The reaction was quenched with water and extracted with ethyl acetate (twice). The organic phase was separated, combined and dried by passing through a hydrophobic frit. Evaporation of the organic phase and purification of the oil so formed by silica chromatography (40 g) (ISCO) eluting with 0-100% ethyl acetate:cyclohexane and then 0-10% methanol:ethyl acetate then methanol gave a gum. This gum was dissolved in methanol (5 mL) and treated with 4N hydrogen chloride in 1,4-dioxane (1 mL) and stirred overnight at room temperature. The reaction mixture was stripped to dryness to give a g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com