Long chain N-alkyl compounds and oxa-derivatives thereof
a technology of nalkyl compounds and imino compounds, which is applied in the direction of biocide, heterocyclic compound active ingredients, amide active ingredients, etc., can solve the problems of high chronic infection rate, increased risk of liver cancer, and no vaccine for those already infected with the virus, so as to reduce, abate, or diminish the virus infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
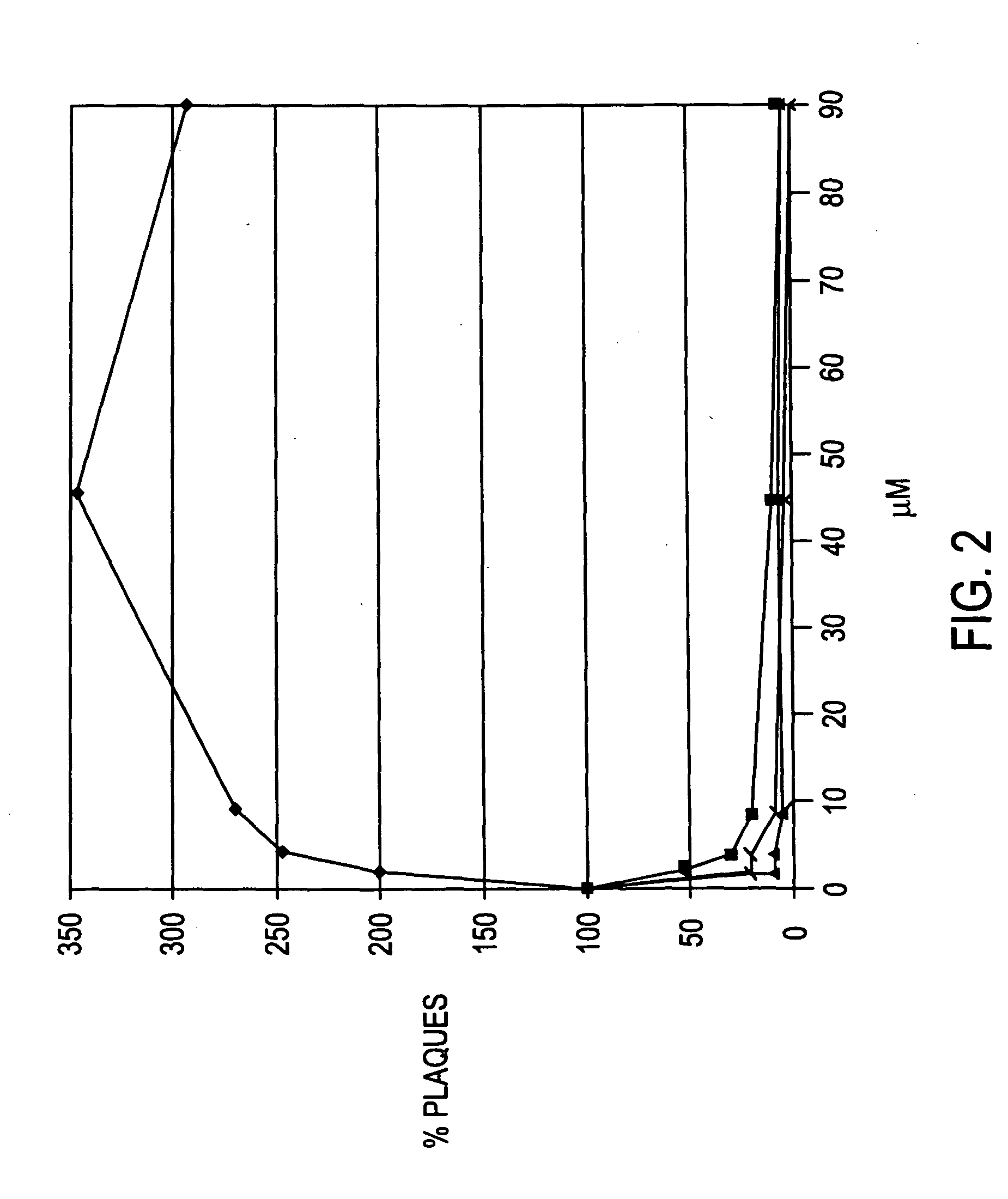
Examples
examples
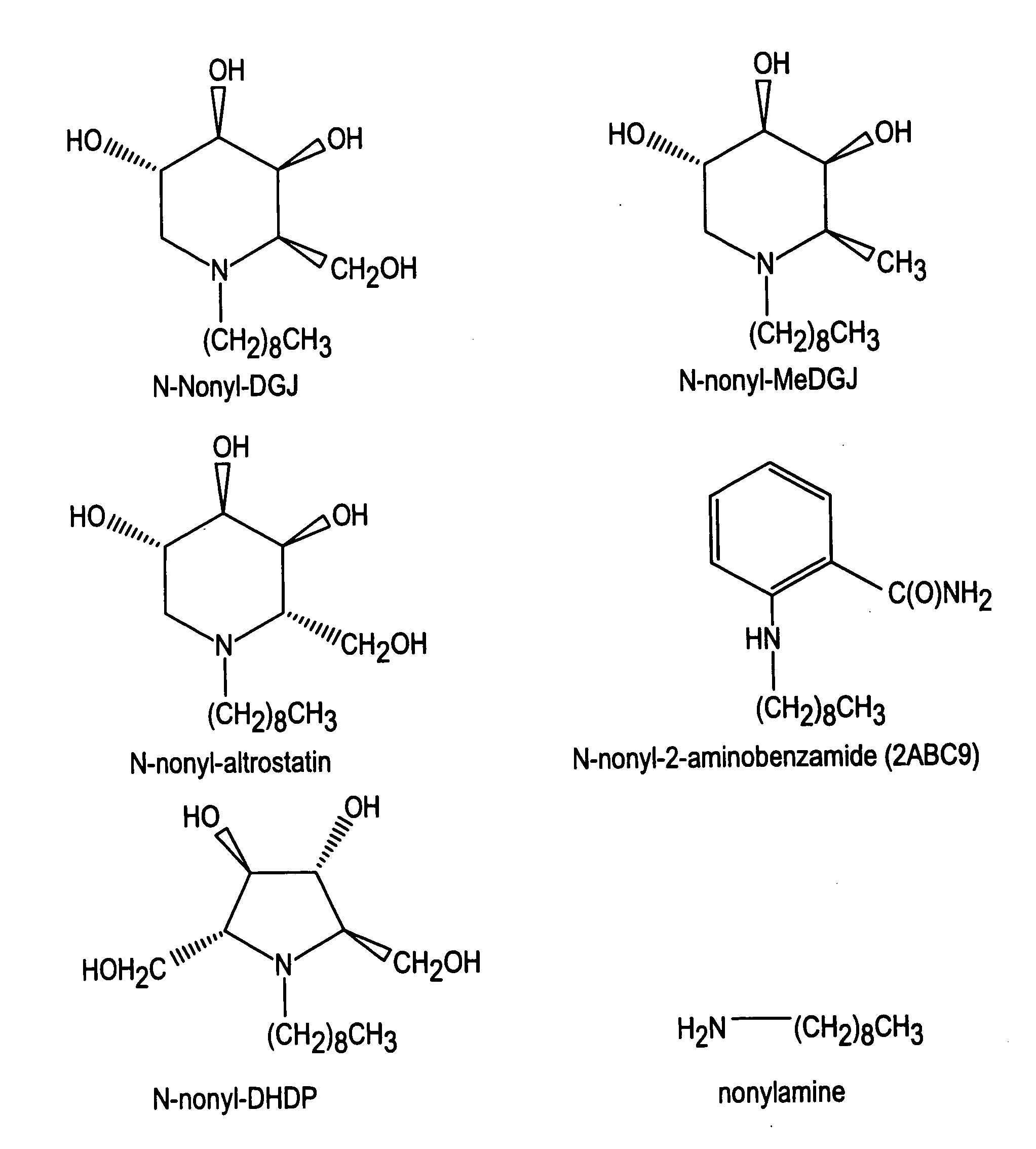
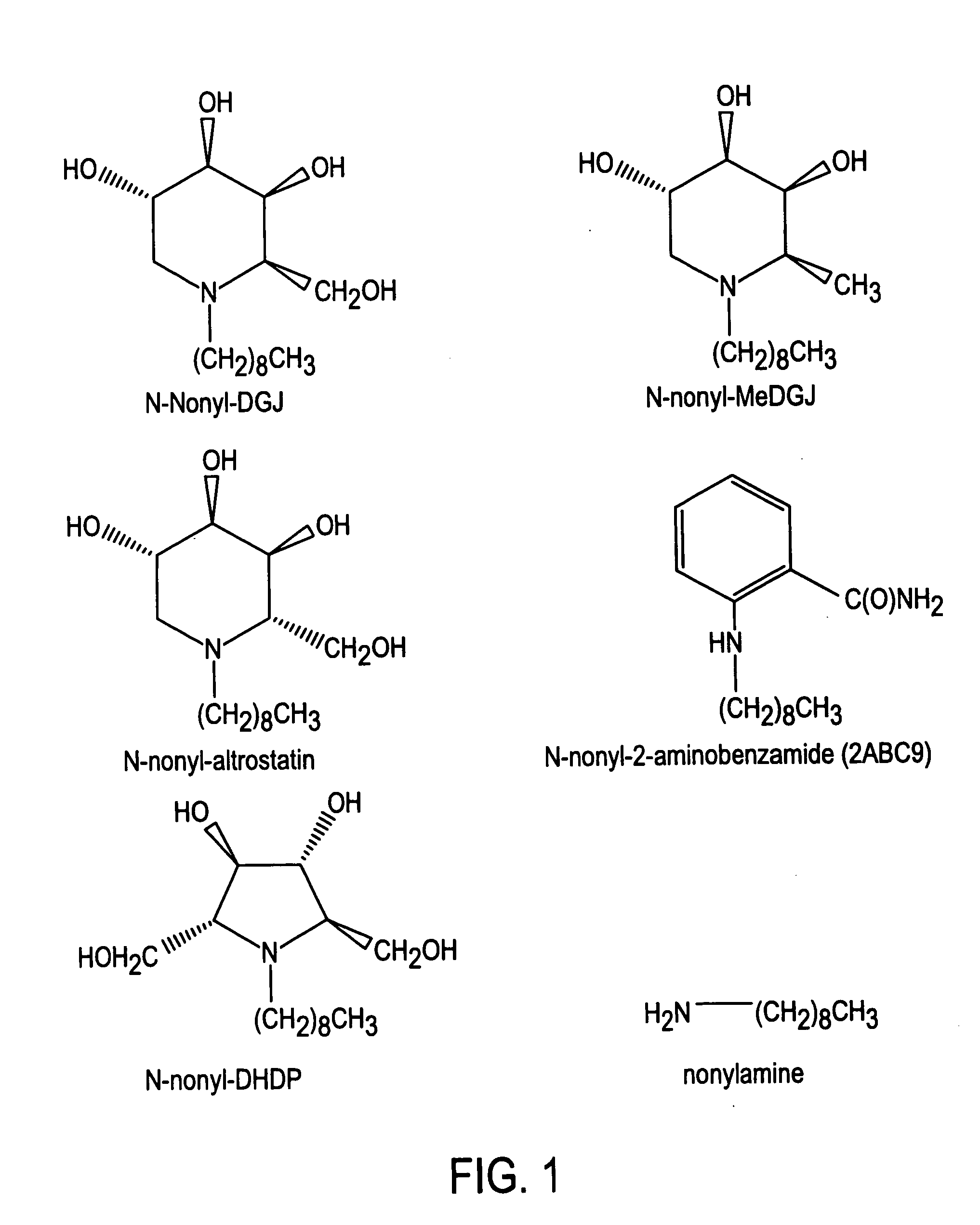
Preparation of N-nonyl-DGJ (NN-DGJ), N-nonyl-methylDGJ (NN-MeDGJ), N-nonyl-altrostatin, N-nonyl-DNJ (N,N-DNJ), N-nonyl-DMDP (N,N-DMDP), and N-nonyl-2-aminobenzamide
[0057]The parent amino or imino compound (DGJ, MeDGJ, altrostatin, DNJ, DMDP, or 2-aminobenzamide (2ABC9) was reductively alkylated with nonylaldehyde (1.2 mol equivalents) in the presence of one mole equivalent of sodium cyanoborohydride for three hours at room temperature in acidified methanol. Typical yields from this reaction were greater than 95% as determined by amperometric detection after high performance cation-exchange chromatography (Dionex). N-Nonyl-compounds were purified from the reaction mixture by high performance liquid chromatography (HPLC) as follows. A sample was applied to a SCX cation-exchange column (7.5×50 mm) in 20% (v / v) acetonitrile and eluted with a linear gradient of 20% acetonitrile containing 500 mM ammonium formate, pH 4.4. The N-nonyl compound was recovered and applied to a C18 reverse-pha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com