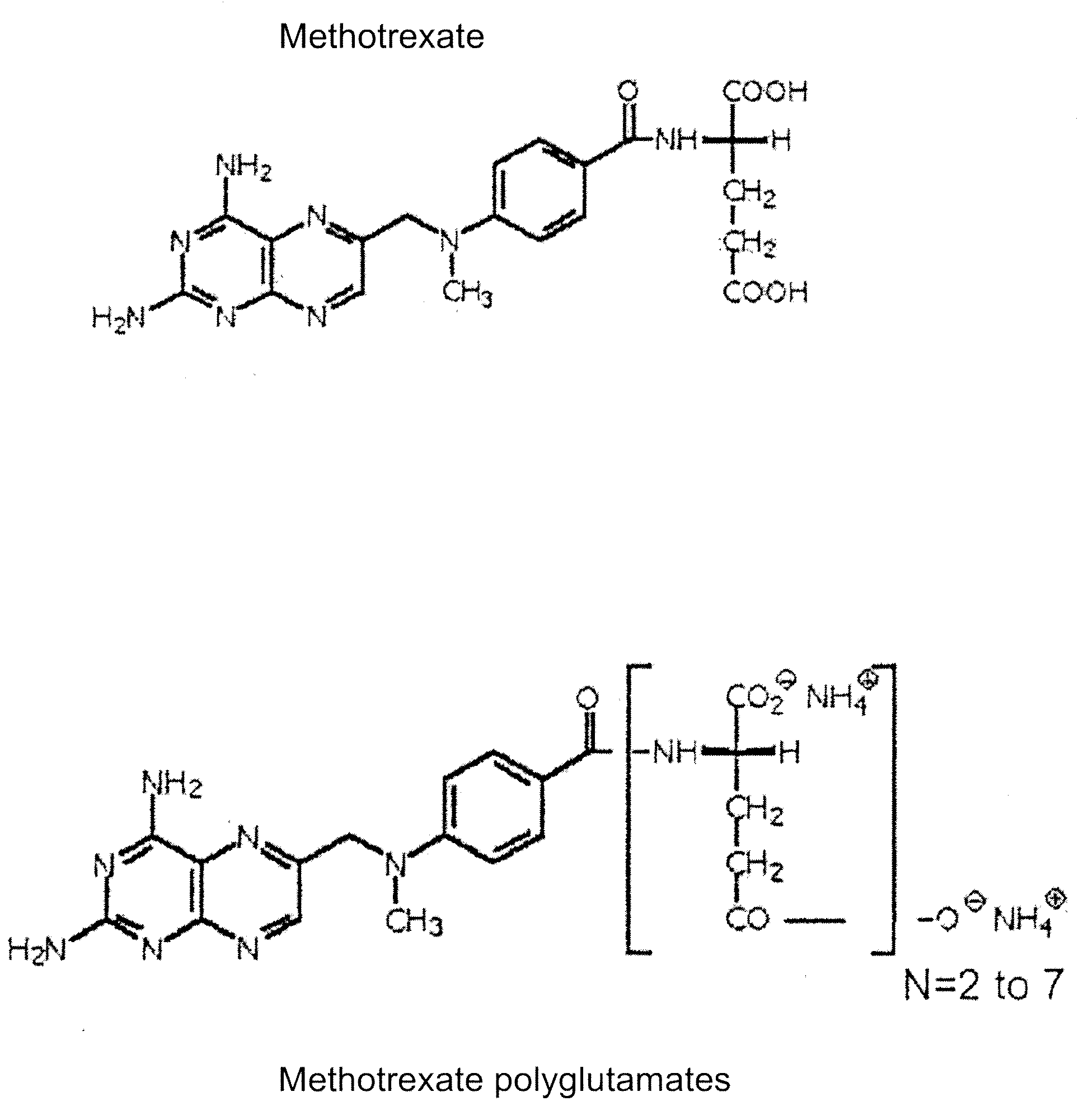
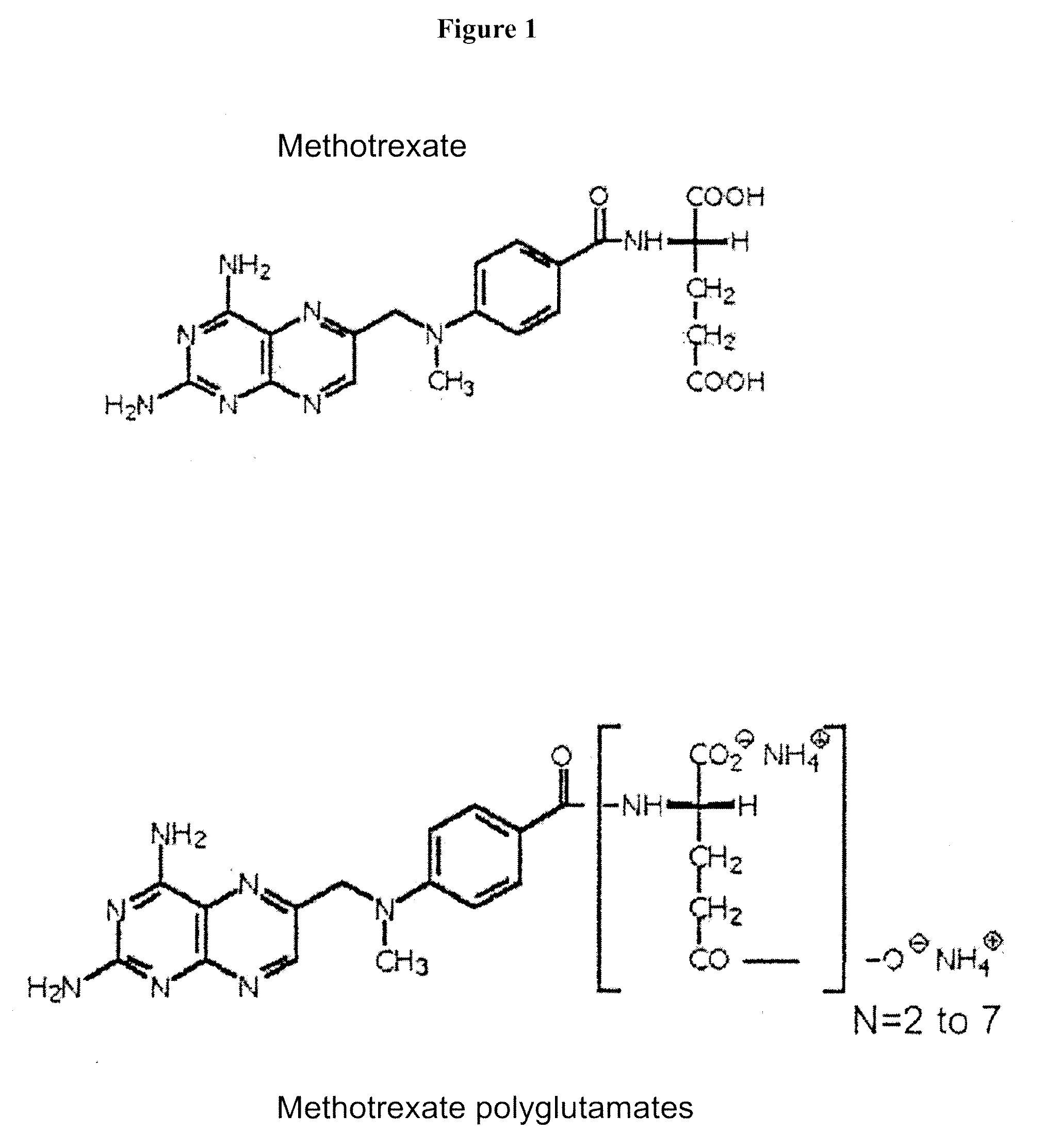
Methods of quantifying methotrexate metabolites
a technology of methotrexate and metabolites, which is applied in the field of monitoring the efficacy and toxicity of antifolate drug therapy, can solve the problems of destroying active proliferating non-target tissues, toxicity of methotrexate treatment, and presenting a risk to patients, so as to optimize the therapeutic effect and reduce toxicity , the effect of optimizing the therapeutic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
[0100]Quantification of Methotrexate by Fluorimetric Detection of Methotrexate-Photolytic Products
[0101]This example describes quantification of methotrexate using high performance liquid chromatography (HPLC) and post-column photolysis followed by fluorometric detection of photolytic products.
[0102]The MTX detection system was based on an HPLC system (Agilent 1100 HPLC chemstation system) equipped with a C18 reversed phase column (25 cm×4.6 mm X Terra MS C18, 5 micrometer particle size; Waters, Milford, Mass.), a post-column photochemical reactor unit (Aura Industries; New York, N.Y.) and a post-reactor unit fluorometer detector. The system also included a C18 pre-column that was changed every 200 injections.
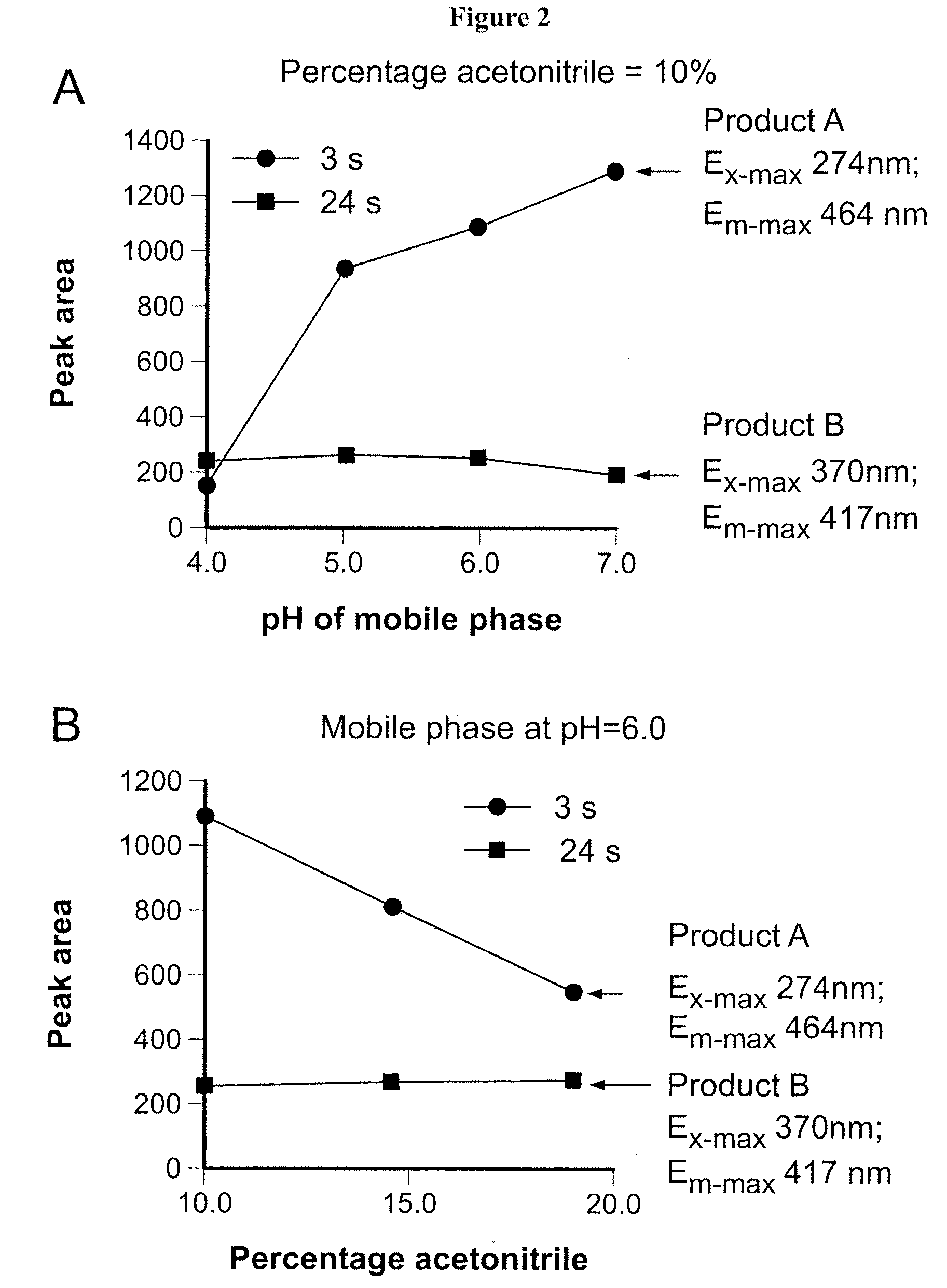
[0103]Samples containing 20 to 1000 nmol / L MTX (Sigma, St. Louis, USA) in RBC extracts were run in the HPLC system at a flow rate of 1 ml / min, with a 15-minute linear gradient from 2% acetonitrile / 98% mobile phase A (10 mM ammonium acetate, pH 6.5, unless otherwise indicated an...
example ii
Conversion of Methotrexate Polyglutamates to Methotrexate
[0110]This example describes efficient conversion of methotrexate polyglutamates (MTXPGs) to methotrexate (MTX) and further demonstrates quantitation of methotrexate polyglutamates in a cellular extract.
[0111]MTXPGs were enzymatically converted to MTX by incubation with RBC extract and plasma as follows. RBCs were isolated from healthy donors (Blood bank; San Diego, Calif.). Hemolysates were prepared having 0.88×109 RBC per 100 μl. For spiked samples, MTXPG (Schirks Laboratories; Jona, Switzerland) was added to RBC extracts at a final concentration of 1 μM (from stock solutions containing 100 μM MTXPG in 0.1 N potassium hydroxide). Reconstituted plasma was prepared from lyophilized plasma (Sigma, St. Louis, USA; Cat. No. P9523) and 100 μl was added to 50 μl of RBC extract, mixed for 30 seconds, and 100 μl of buffer containing 100 mM potassium phosphate pH 4.5 and 150 mM mercaptoethanol was added. Because the reconstituted plas...
example iii
Monitoring Methotrexate Polyglutamate Levels in Patients Receiving Low-Dose Methotrexate Therapy
[0118]Methotrexate requires intracellular activation to methotrexate polyglutamate to exert anti-arthritic effects. This example describes a method for quantitating the levels of intracellular methotrexate polyglutamates in the RBCs of a patient following administration of low-dose MTX therapy.
[0119]Blood samples (5 ml) from polyarthritis patients receiving low-dose MTX were collected after written informed consent. The patients had received a median of 15 mg MTX (range 2.5 mg to 40 mg per week) for at least three months. Blood samples were centrifuged for 10 minutes to separate plasma and buffy coat from RBCs. After washing the RBC pellet with 2 volumes of saline, RBC count was determined with a Beckman Coulter Onyx Counter; RBCs were subsequently stored at −70° C. until analysis. Results were normalized to 10 red blood cells. Patient results were expressed as average ±SEM (range).
[0120]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com