Pharmaceutical composition formulated for pre-gastric absorption of monoamine oxidase b inhibitors
a technology of monoamine oxidase and pre-gastric absorption, which is applied in the direction of drug compositions, biocide, nervous disorders, etc., can solve the problems of inability to absorb intact selegiline, the inability to reduce the bioavailability of selegiline administered in the conventional manner, and the inability to achieve significant absorption of intact selegiline, etc., to avoid the disadvantage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of a Fast-Dispersing Dosage Form Comprising Selegiline
[0062](a) Preparation of Selegiline Hydrochloride 2.0% Dispersion
[0063]Gelatin (720 g) and mannitol (540 g) were dispersed in a portion of purified water (15.75 kg) by mixing thoroughly in the bowl of a vacuum mixer. The remaining water (1.5 liters) was added under vacuum while mixing using an anchor stirrer. The mix was then heated to 40° C.±2° C. and homogenized for ten minutes. When cooled, a 4500 g portion of the mix was removed into a stainless steel vessel and glycine (360 g) aspartame (90 g), grapefruit flavor (54 g), Opatint™ yellow (54 g), citric acid (90 g) and selegiline hydrochloride (360 g) were then added sequentially to this portion while homogenizing using a bench top homogenizer. The remainder of the mix was transferred into a second stainless steel vessel. The mix was homogenized for ten minutes using a bench top homogenizer to dissolve the drug. Once dispersion of the coloring agent was complete, th...
example 2
Comparative Pharmacokinetic Study of Selegiline Dosage Forms
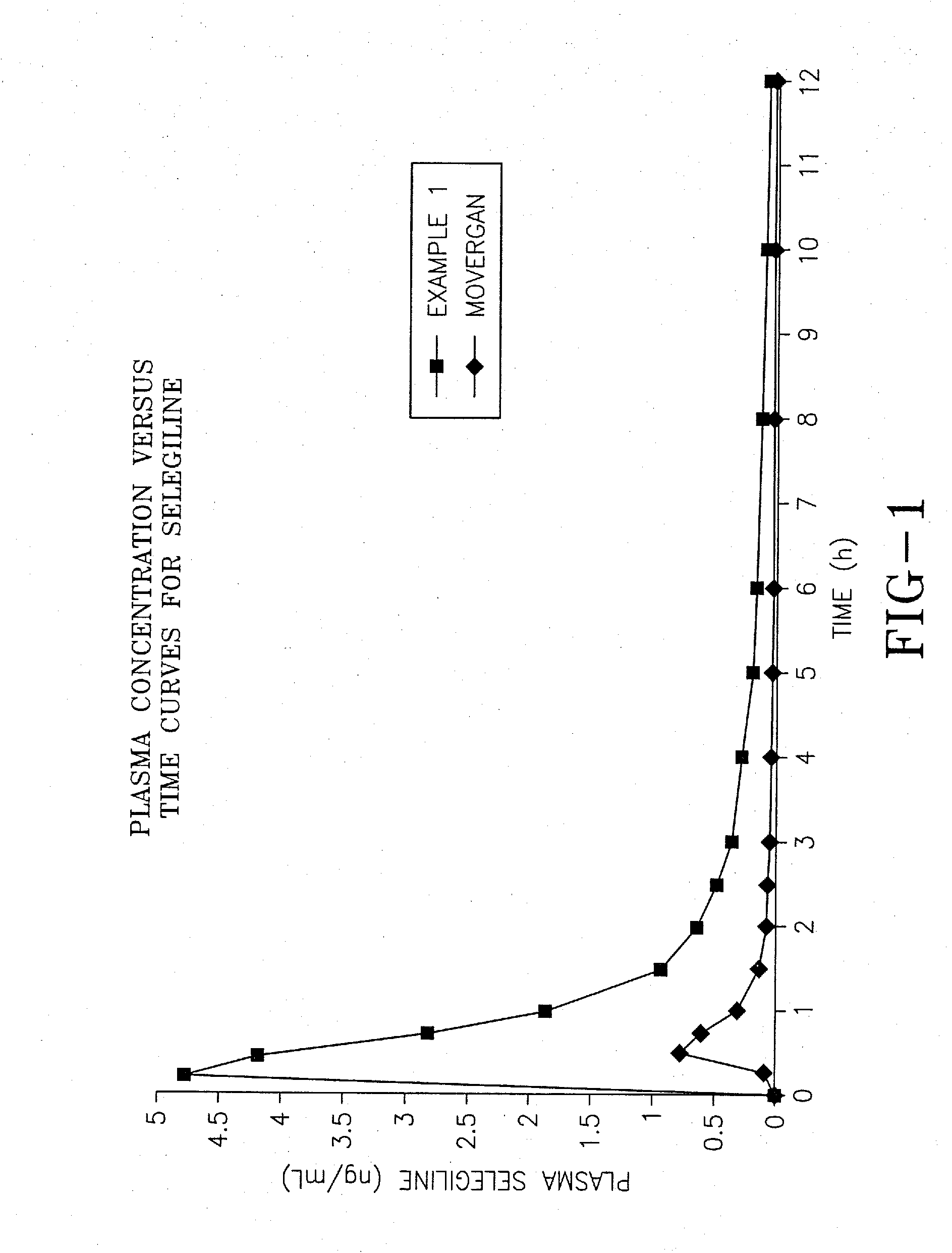
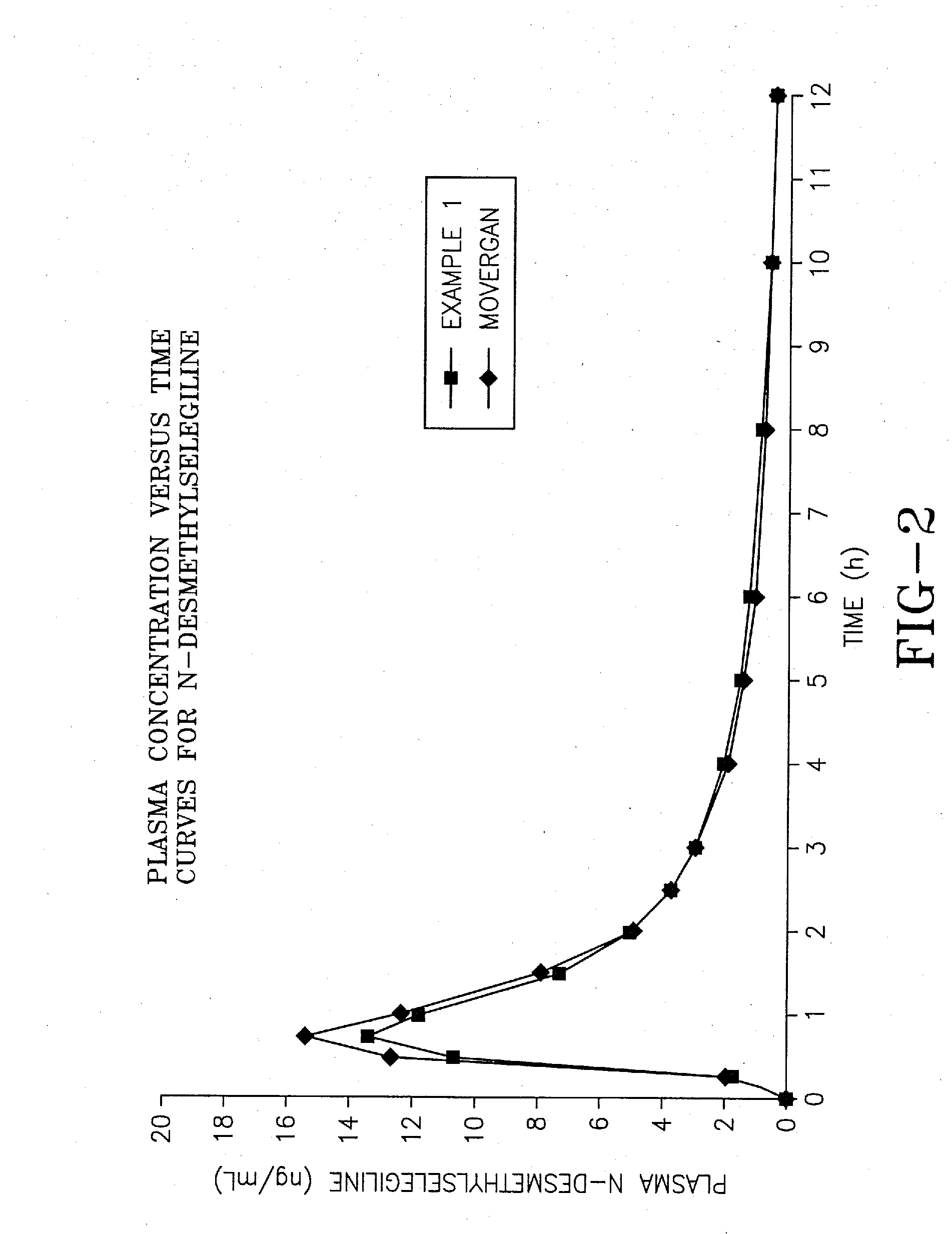
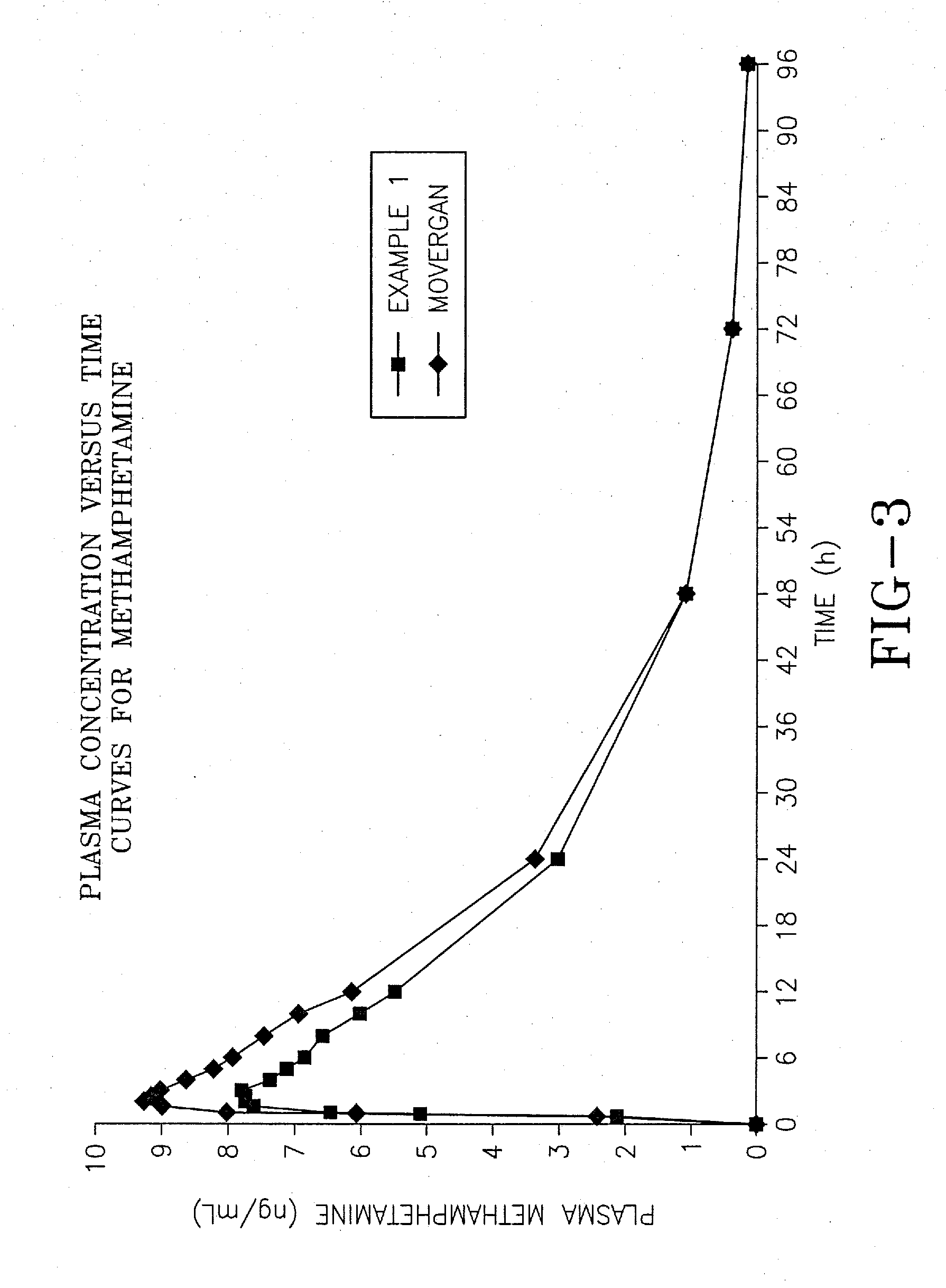
[0067]The aim of this experiment was to compare the bioavailability of the selegiline hydrochloride formulation of Example 1 prepared according to the invention, with the commercially available tablet form of selegiline hydrochloride sold under the trademark Movergan™ (available from Asta Medica AG, Weismullerstrasse 45, 6000 Frankfurt am Main, Germany).
[0068]An open label, randomized, 2-way crossover, volunteer study was performed as follows. Twenty-four subjects of either sex, aged between 45 and 71 years, giving written informed consent, underwent a thorough medical examination to establish their fitness to participate in the study. Subjects received study treatment in the order dictated by a pre-determined randomization schedule. Subjects were given either the formulation of Example 1, or the Movergan™ formulation. Blood samples for determination of pharmacokinetic parameters were taken at baseline (immediately before d...
example 3
Pre-Gastric Absorption Study
[0075]The aim of this study was to assess the sublingual absorption of selegiline hydrochloride formulations according to Example 1. The pharmacokinetic profile of selegiline hydrochloride from the commercially available US tablet formulation should under the registered trademark Eldepryl® (available from Somerset Pharmaceuticals, Inc. 777 South Harbor Island Boulevard, Suite 880, Tampa Fla. 33602) served as a control for the degree of gastrointestinal absorption of selegiline. In addition, the study was designed to compare the urinary excretion over 24 hours of phenethylamine and 5-hydroxyindoleacetic acid (5-HIAA) from the subjects to whom such formulations had been administered.
[0076]This study was an open-labeled randomized 3-way crossover volunteer study and was performed as follows:
[0077]Eleven subjects of either sex aged between 45 and 62 years giving written informed consent underwent a thorough medical examination to establish their fitness to pa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time period | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com