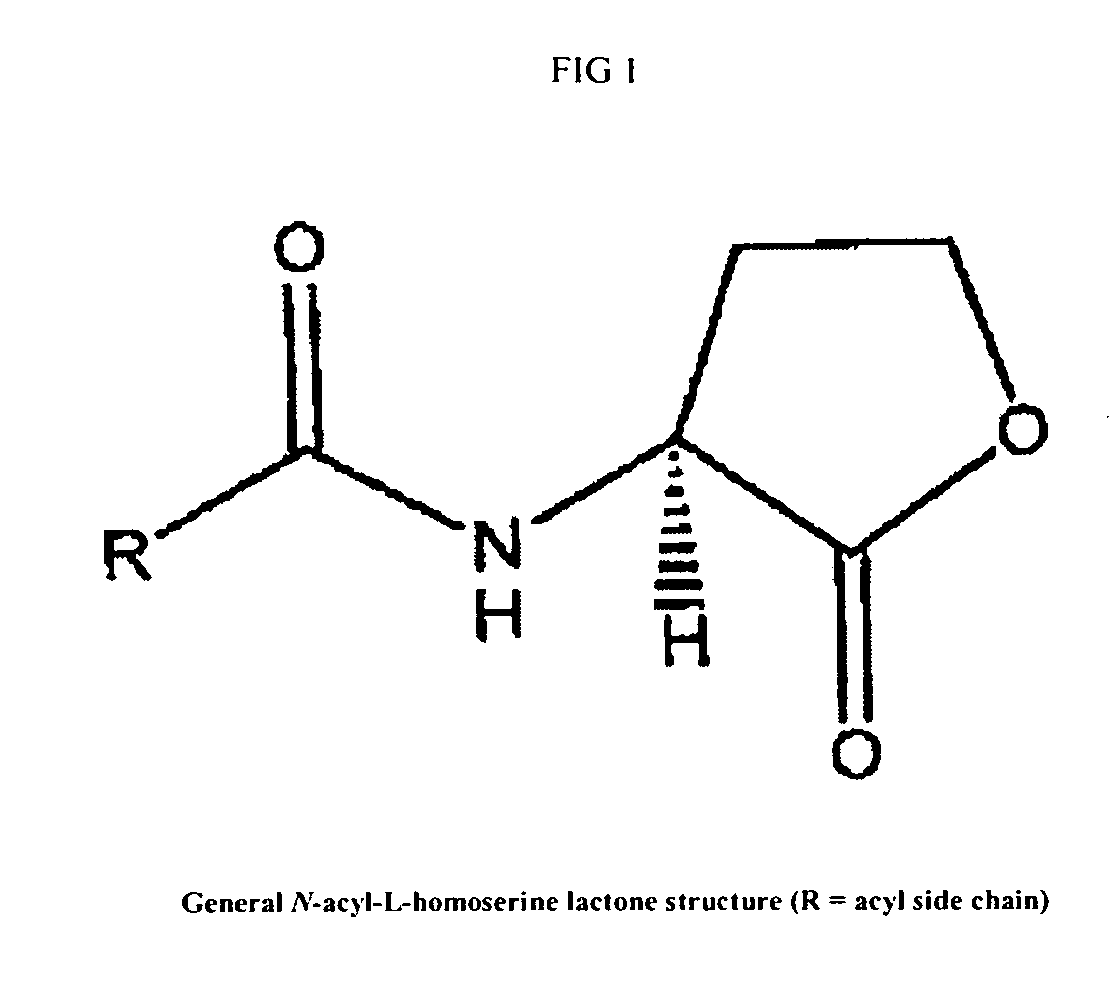
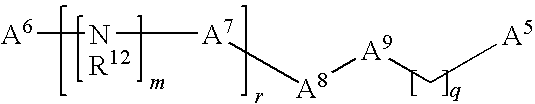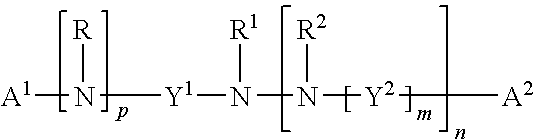Modulation of pathogenicity
a pathogenicity and module technology, applied in the field of module of pathogenicity, can solve the problems of inability to identify rational drug design targets, severe damage or diseases of algae in different areas, and huge costs in public health systems worldwide, and achieve the effects of reducing virulence gene expression, swarming motility and biofilm formation, and modulating bacterial cell-cell communication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
1. Synthesis of Compounds of Formula (XIII) (β-Ketoamides)
[0099]The acyl Meldrum's acid (1.2 eq) was dissolved in anhydrous benzene (concentration approximately 0.4 mol / l), and the amine (1.0 eq) was added. In case of amine hydrochlorides, one equivalent of triethylamine or N,N-diisopropylethylamine was added. The mixture was refluxed until tlc showed complete conversion (typically, 4 to 6 h). The benzene solutions were directly chromatographed on silica gel in an appropriate solvent mixture (isohexane-ethyl acetate, dichloromethane-methanol, or dichloromethane-acetonitrile mixtures). Yields of the purified products typically were in the range from 30 to 75%.
[0100]In the following Table 2, the synthesis method employed in each case for the respective compound or whether the compound was obtained is indicated. Furthermore, the mass found by LC / (+)-ESI and LC / (−)-ESI mass spectrometry, the molecular mass, the NMR data (300.13 MHz, residual solvent peaks were used as internal standards...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com