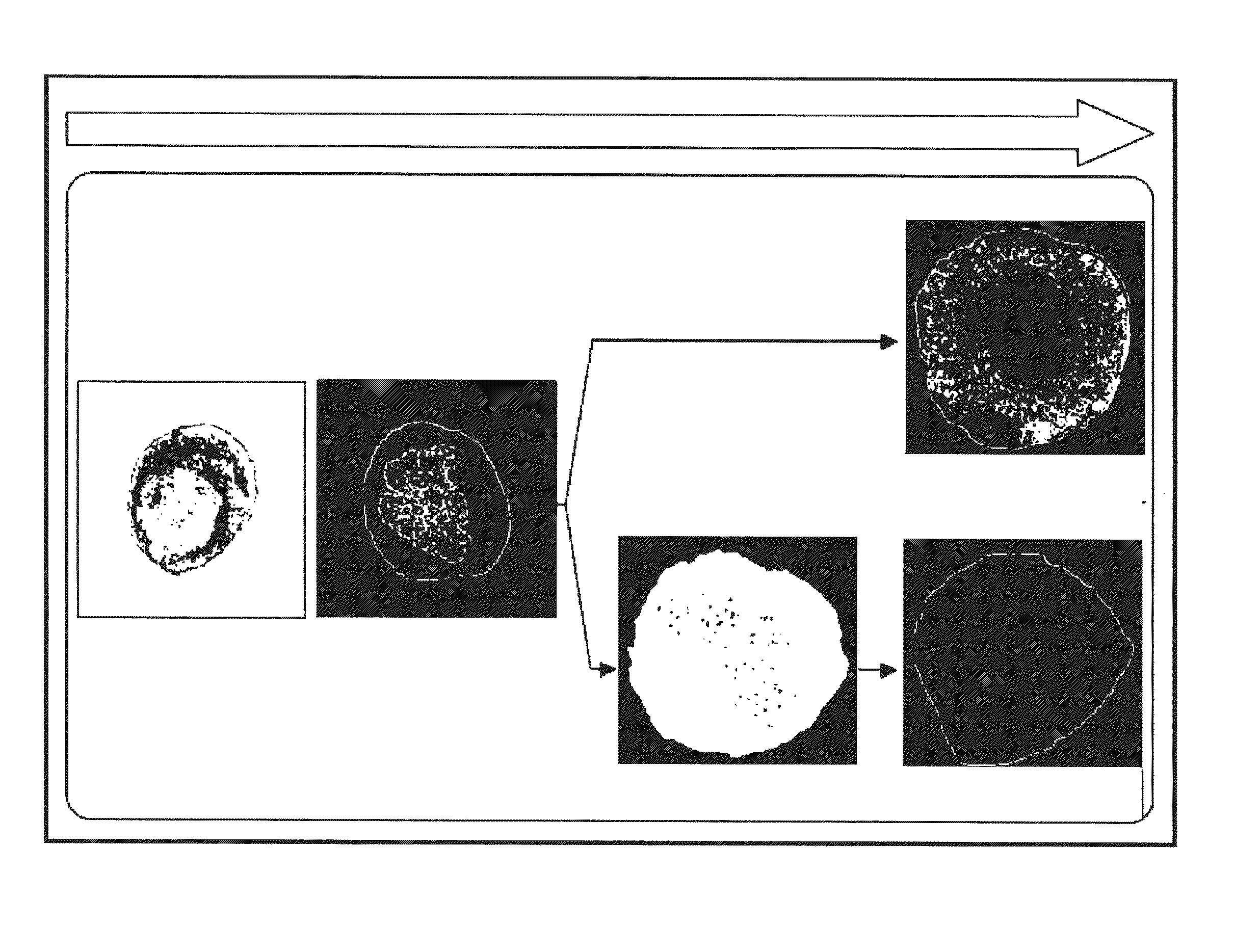
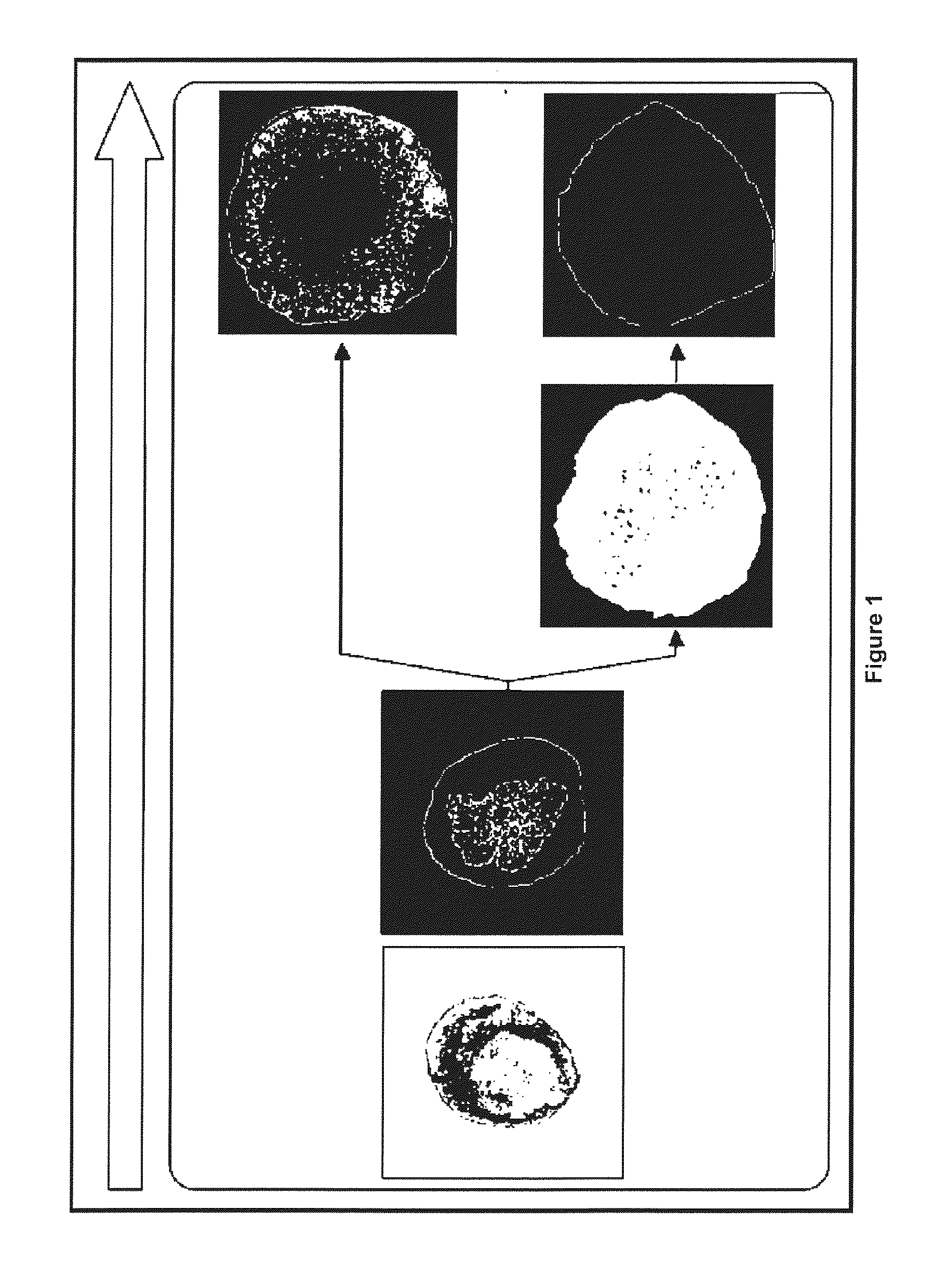
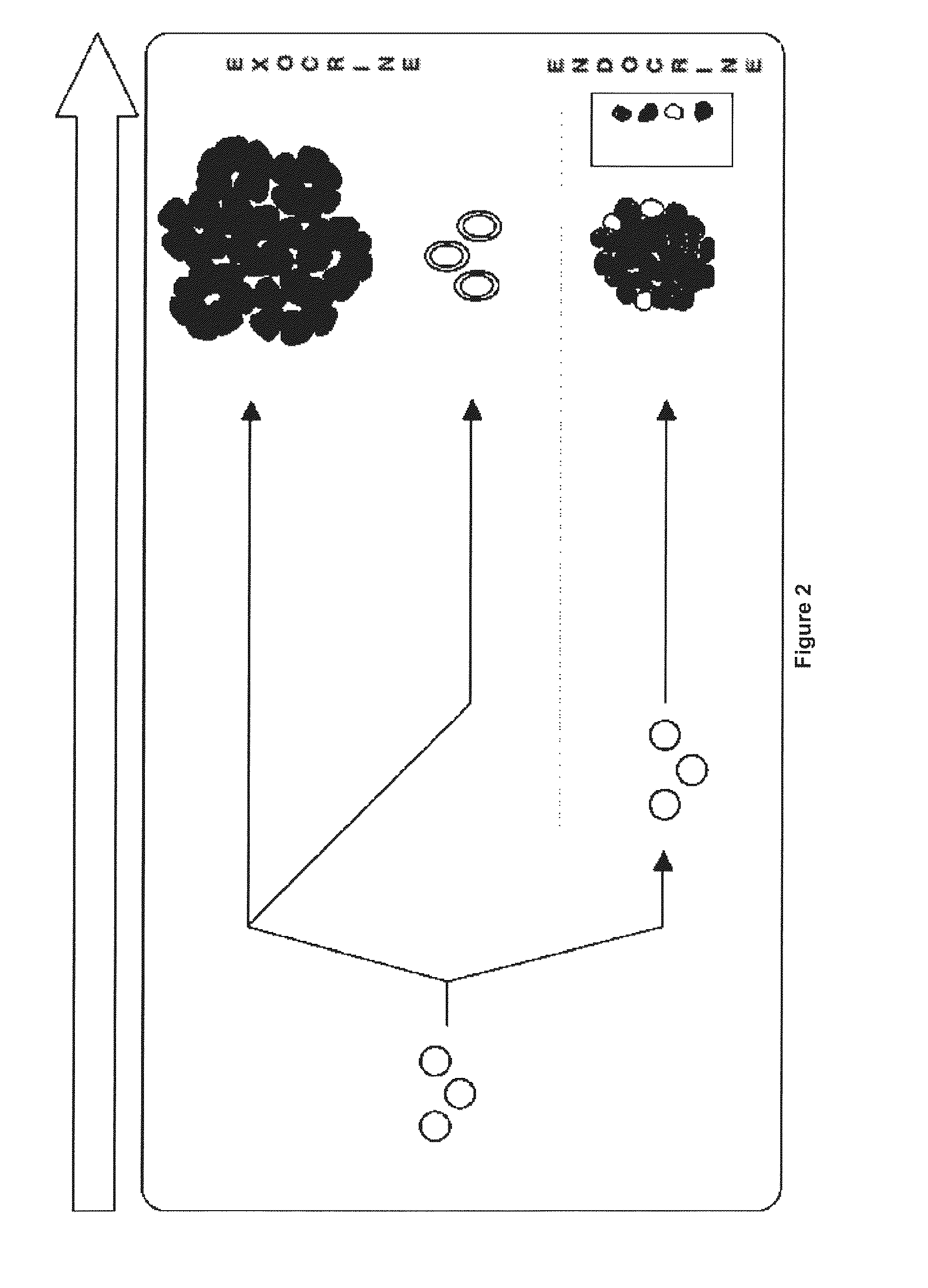
Method for obtaining ngn3-expressing cells and insulin producing-beta cells
a technology of ngn3 and beta cells, which is applied in the field of obtaining ngn3expressing cells and insulin producingbeta cells, can solve the problems of poor reproducibility of bone marrow, lack of organ donors, and methods that are really satisfying
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Material & Methods
[0117]Animals and dissection of dorsal pancreatic rudiments: Pregnant Wistar rats were purchased from the CERJ (Le Genest, France). The first day post-coitum was taken as embryonic day 0.5 (E0.5). Pregnant female rats at 13.5 days of gestation were killed by CO2asphyxiation, according to the French Animal Care Committee's guidelines. Dorsal pancreatic buds from E13.5 rat embryos were dissected as described previously (2).
[0118]Organ culture, HDACi treatments, BrdU incorporation: Pancreases were laid on 0.45 μm filters (Millipore) at the air-medium interface in Petri dishes, containing RPMI 1640
[0119](Invitrogen) supplemented with penicillin (100 U / mL), streptomycin (100 μg / mL), HEPES (10 mmol / L), L-glutamine (2mmol / L), non-essential amino acids (1x, Invitrogen) and 10% heat-inactivated calf serum (HyClone). Cultures were maintained at 37° C. in humidified 95% air / 5% CO2. Medium was changed every other day. Explants were cultured in the presence of VPA or TSA (Sigma...
example 2
[0126]In vivo HDACi injections in rat: Experiments were submitted for ethical evaluation to the “Comité Régional d′Ethique pour l′Experimentation Animale Ile-de-France-Paris Descartes” and approved (register number P2.CHS.081.09). Pregnant rat wistar females are injected intraperitoneally daily with HDACi between E13.5 and E20.5. The NaB treated group received 1000 mg / kg whereas the VPA treated group received 300 mg / kg, in Phosphate Buffer Saline solution (PBS). After 7 days of treatment, the animals are killed by CO2 asphyxiation, according to the French Animal Care Committee's guidelines. Embryos are dissected and each pancreas is fixed or frozen for subsequent analysis by immunohistochemistry or western-blot respectively.
example 3
[0127]In vitro HDACi treatment of human pancreas: Human pancreases are extracted from foetal tissue fragments obtained immediately after voluntary abortions performed around 9 weeks of development, in compliance with the current French legislation. Human pancreases are cultured in the same conditions described above used for rat pancreases, and treated until 14 days with 800nM TSA.
Results
[0128]HDACs are down-regulated during pancreas development: The inventors first analyzed the expression of different HDACs during rat pancreas development. Using Western blotting, we found that both class I (HDAC1-3) and class II (HDAC4-7) HDACs were expressed at E13.5, E17.5 and in the adult pancreas. The expression levels of most HDACs (with the exception of HDAC3) decreased during development. The inventors next measured total HDAC activity and observed a 86.1% ±6.5% decrease at E17.5 compared with E13.5. To determine whether decreased HDAC activity correlated with increased histone acetylation, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com